Answered step by step
Verified Expert Solution
Question
1 Approved Answer
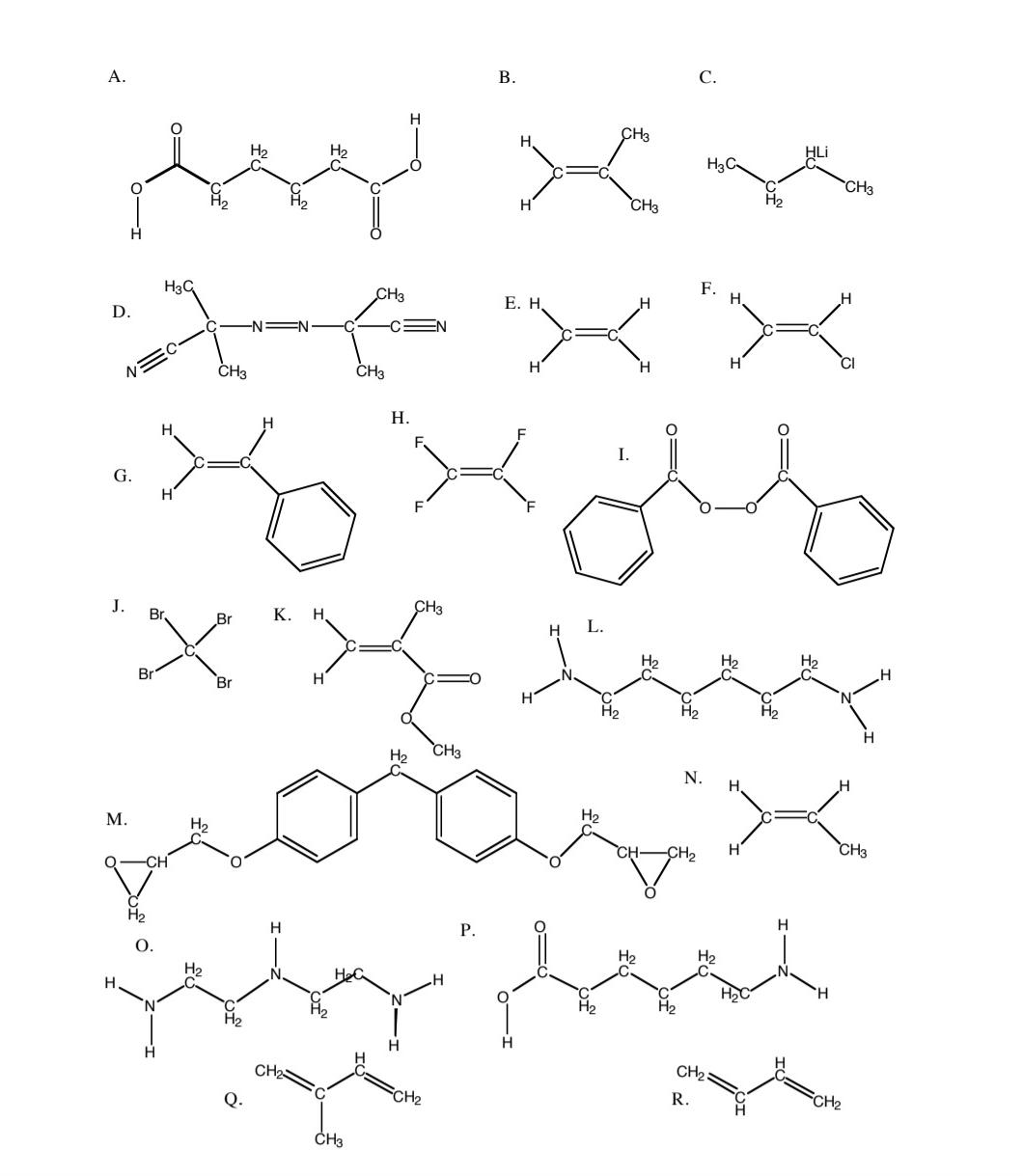
You oversee a polymer synthesis laboratory which only has the chemicals labelled A through R below. a . Which molecule could be used as an
You oversee a polymer synthesis laboratory which only has the chemicals labelled A through below.
a Which molecule could be used as an initiator for a free radical polymerization? Write the chemical reactions representing initiation as well as a differential equation for the rate of generation of free radicals. What does depend on besides the rate constants for the chemical reactions
b You want to produce polystyrene PS What molecule should you use as the monomer? Draw the propagation reactions.
c Write two chemical reactions which are possible termination reactions for the growing PS radicals. Write a differential equation for the rate of disappearance of PS radicals. What is the dependence of this rate on the concentration of PS radicals,
d What is the "steady state" approximation? Use this approximation with your answers to a and c to find : as a function of the initiator concentration, I
e What additional information will you need to find the kinetic chain length? Assuming that information is available, show how you would use it to determine the kinetic chain length.
A
B
C
F
H
J
K
N
P
Q
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


