Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You receive a Pt/Al2O3 catalyst of unknown surface area to be used for dehydrogenating cyclohexane to benzene. You think that the pores of the
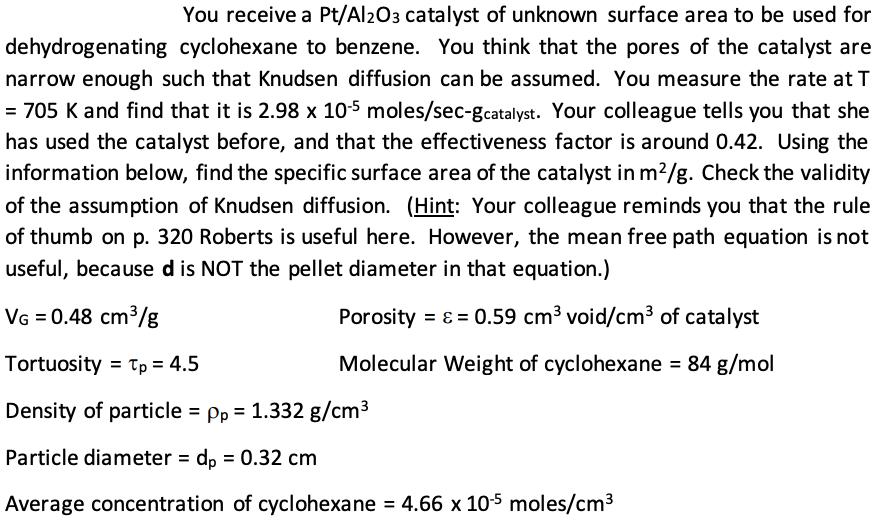
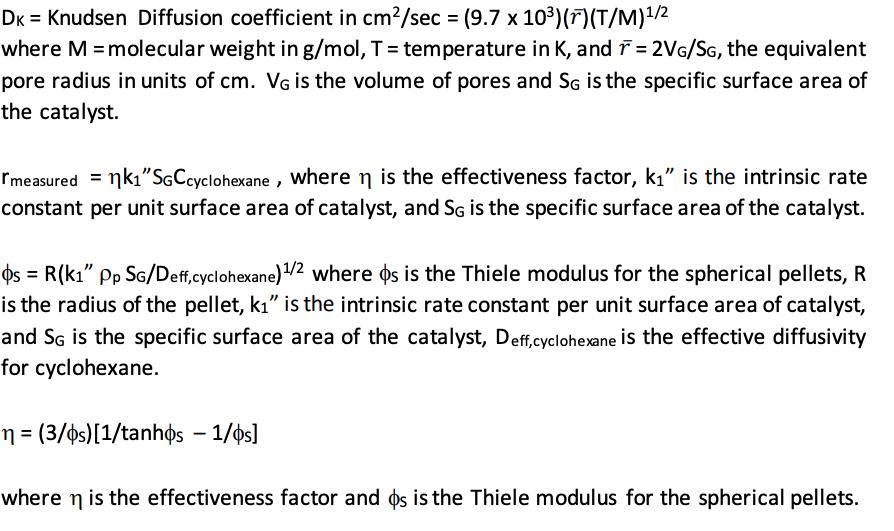
You receive a Pt/Al2O3 catalyst of unknown surface area to be used for dehydrogenating cyclohexane to benzene. You think that the pores of the catalyst are narrow enough such that Knudsen diffusion can be assumed. You measure the rate at T = 705 K and find that it is 2.98 x 10-5 moles/sec-gcatalyst. Your colleague tells you that she has used the catalyst before, and that the effectiveness factor is around 0.42. Using the information below, find the specific surface area of the catalyst in m/g. Check the validity of the assumption of Knudsen diffusion. (Hint: Your colleague reminds you that the rule of thumb on p. 320 Roberts is useful here. However, the mean free path equation is not useful, because d is NOT the pellet diameter in that equation.) VG = 0.48 cm/g Porosity = = 0.59 cm void/cm of catalyst Molecular Weight of cyclohexane = 84 g/mol Tortuosity Tp = 4.5 Density of particle = pp = 1.332 g/cm Particle diameter = dp = 0.32 cm Average concentration of cyclohexane = 4.66 x 10-5 moles/cm DK = Knudsen Diffusion coefficient in cm/sec = (9.7 x 10) (r) (T/M)/2 where M = molecular weight in g/mol, T = temperature in K, and T = 2VG/SG, the equivalent pore radius in units of cm. VG is the volume of pores and SG is the specific surface area of the catalyst. Imeasured = k"SGCcyclohexane, where n is the effectiveness factor, k" is the intrinsic rate constant per unit surface area of catalyst, and SG is the specific surface area of the catalyst. os= R(k1" Pp SG/Deff, cyclohexane) /2 where ps is the Thiele modulus for the spherical pellets, R is the radius of the pellet, k" is the intrinsic rate constant per unit surface area of catalyst, and SG is the specific surface area of the catalyst, Deff, cyclohexane is the effective diffusivity for cyclohexane. n = (3/os)[1/tanhos - 1/os] where n is the effectiveness factor and ps is the Thiele modulus for the spherical pellets.
Step by Step Solution
★★★★★
3.28 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


