Answered step by step
Verified Expert Solution
Question
1 Approved Answer
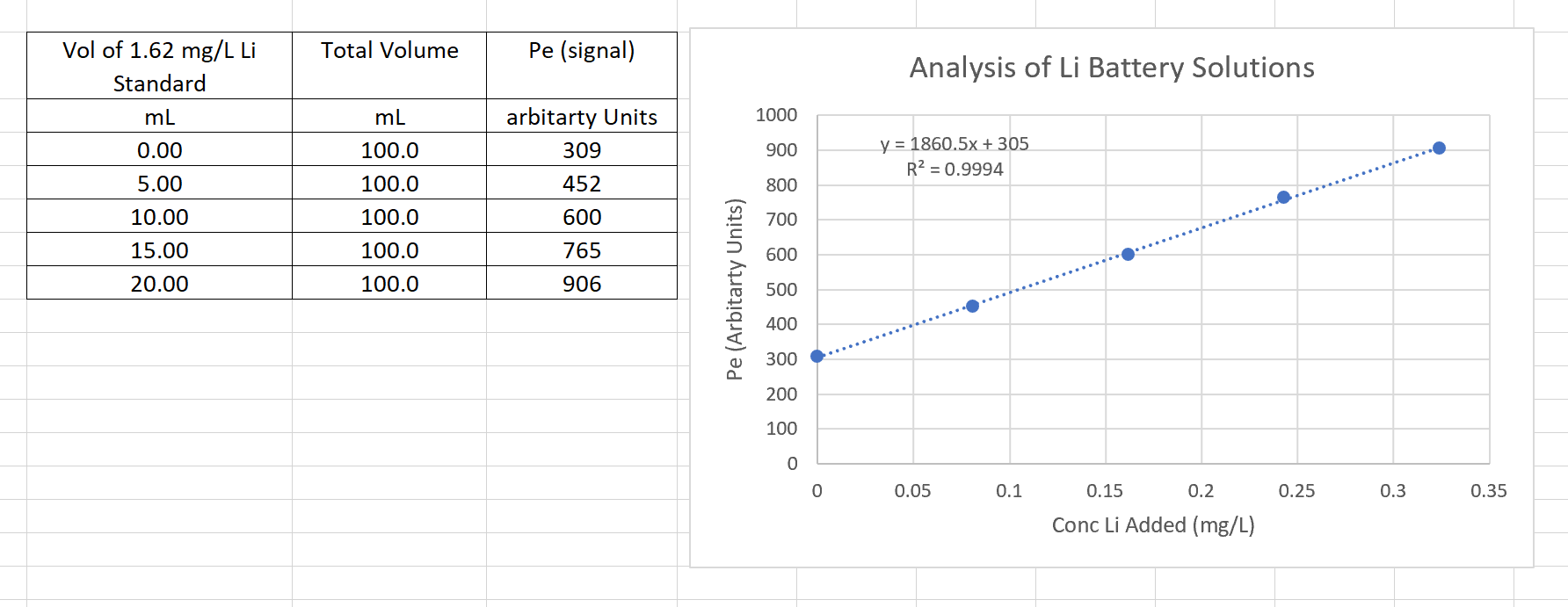
You received a job in a company that recycles Li batteries. You are analyzing the Li solutions by flame emission. The matrix of the batteries
You received a job in a company that recycles Li batteries. You are analyzing the Li solutions by flame emission. The matrix of the batteries is quite complex and the spike recoveries keep failing. The Li concentration was determined by atomic emission using the method of standard additions.
To each the five mL flask mL of Libearing battery solutionsample was added. Aliquots of a mgL Li standard solution was added to each flask, see table below. Each flask was diluted to the mark with DI water.
From the data and the following graph, find the concentration of Li in the unknown Libearing battery solutionsample in ppm Report your answer to decimal points and no units.
tabletableVol of mgL LiStandardTotal Volume,Pe signalarbitarty Units
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


