Answered step by step
Verified Expert Solution
Question
1 Approved Answer
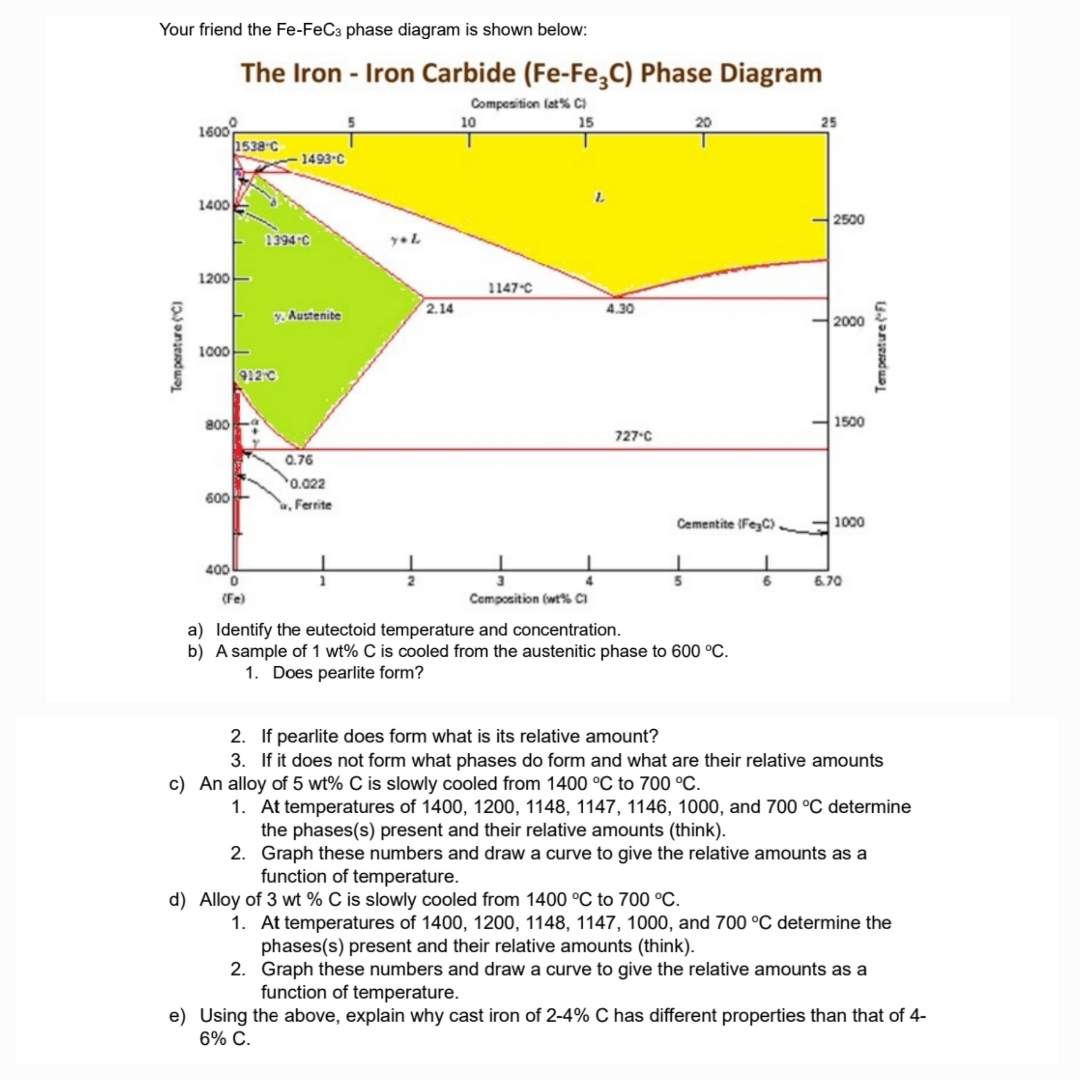
Your friend the F e - F e C 3 phase diagram is shown below: b ) A sample of 1 w t % C
Your friend the phase diagram is shown below:
b A sample of is cooled from the austenitic phase to
Does pearlite form?
If pearlite does form what is its relative amount?
If it does not form what phases do form and what are their relative amounts
c An alloy of is slowly cooled from to
At temperatures of and determine the phasess present and their relative amounts think
Graph these numbers and draw a curve to give the relative amounts as a function of temperature.
d Alloy of is slowly cooled from to
At temperatures of and determine the phasess present and their relative amounts think
Graph these numbers and draw a curve to give the relative amounts as a function of temperature.
e Using the above, explain why cast iron of has different properties than that of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


