Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Your graduate student studying the stability of anthocyanins at 70 C found the time required for 80% of anthocyanins to disappear, depending on the initial
Your graduate student studying the stability of anthocyanins at 70 C found the time required for 80% of anthocyanins to disappear, depending on the initial concentration, as follows. According to these results, which reaction degree can explain the degradation of anthocyanins and calculate the time required for 80% of anthocyanins to be degraded. 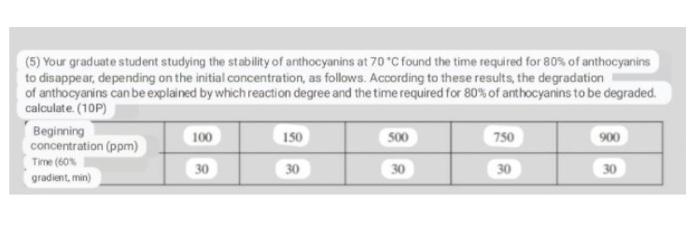
(5) Your graduate student studying the stability of anthocyanins at 70C found the time required for 80$ of anthocyanins to disappear, depending on the initial concentration, as follows. According to these results, the degradation of anthocyanins can be explained by which reaction degree and the time required for 80% of anthocyanins to be degraded 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


