Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider the Ricker population model presented in class, Rt+1 = F(Rt) = aRte aRte-bRt where R is the total mass of the recruits in
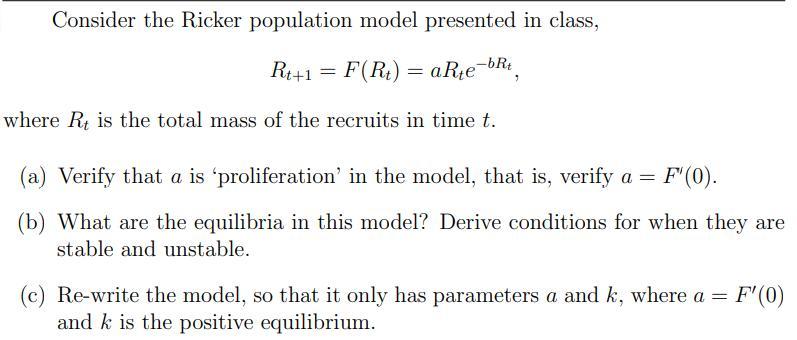
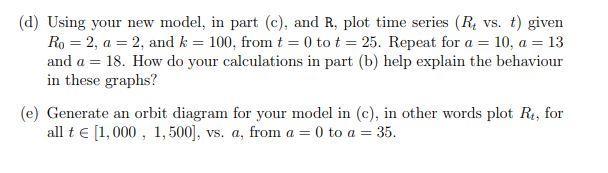
Consider the Ricker population model presented in class, Rt+1 = F(Rt) = aRte aRte-bRt where R is the total mass of the recruits in time t. (a) Verify that a is 'proliferation' in the model, that is, verify a = F'(0). (b) What are the equilibria in this model? Derive conditions for when they are stable and unstable. F'(0) (c) Re-write the model, so that it only has parameters a and k, where a = and k is the positive equilibrium. (d) Using your new model, in part (c), and R. plot time series (R, vs. t) given Ro = 2, a = 2, and k = 100, from t = 0 to t = 25. Repeat for a = 10, a = 13 and a = 18. How do your calculations in part (b) help explain the behaviour in these graphs? (e) Generate an orbit diagram for your model in (c), in other words plot R, for all t [1,000, 1, 500], vs. a, from a = 0 to a = 35.
Step by Step Solution
★★★★★
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
a To verify that a is proliferation in the model we need to calculate the derivative of FR with respect to R at R0 This gives us F0a Therefore a is in...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


