Answered step by step
Verified Expert Solution
Question
1 Approved Answer
zero absorbance or 1 0 0 % . If not, adjust the spectrophotometer accordingly. ( 2 ) Set the wavelength of the spectrophotometer to 4
zero absorbance or If not, adjust the spectrophotometer
accordingly.
Set the wavelength of the spectrophotometer to Place the
stock solution Solution in a cuvet following the "blank solution
procedure" as above. Measure and record the wavelength and
absorbance Scan the wavelength range of the spectrophotometer
while recording absorbance values. You will need to scan manually,
setting the absorbance at zero with the blank solution
at EACH chosen wavelength before recording the
absorbance of the stock solution.
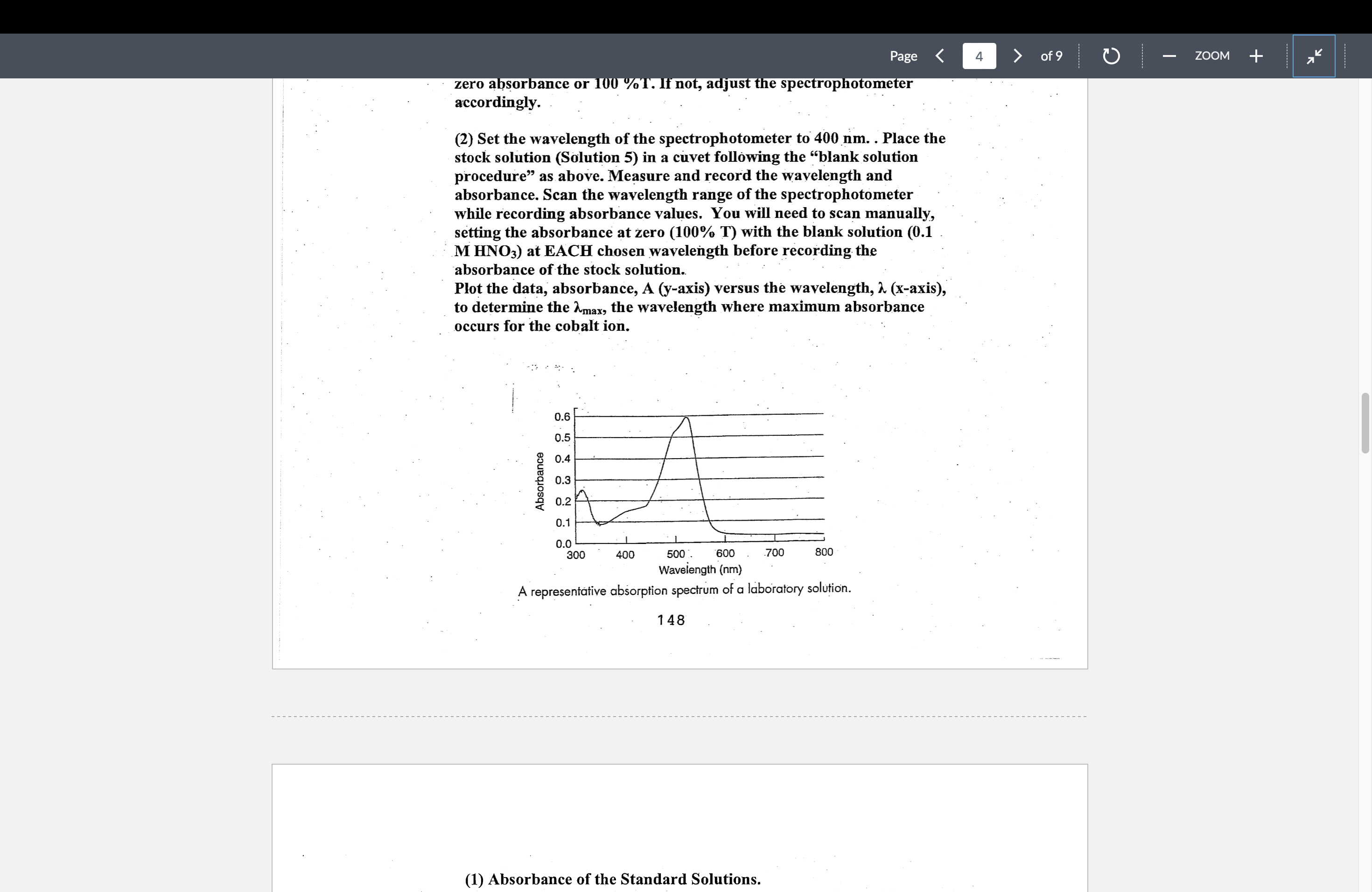
Plot the data, absorbanceaxis versus the wavelength, axis
to determine the the wavelength where maximum absorbance
occurs for the cobalt ion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


