For the thermodynamic cycle shown in Fig. 20-3, find (a) The net work output of the gas
Question:
For the thermodynamic cycle shown in Fig. 20-3, find
(a) The net work output of the gas during the cycle and
(b) The net heat flow into the gas per cycle.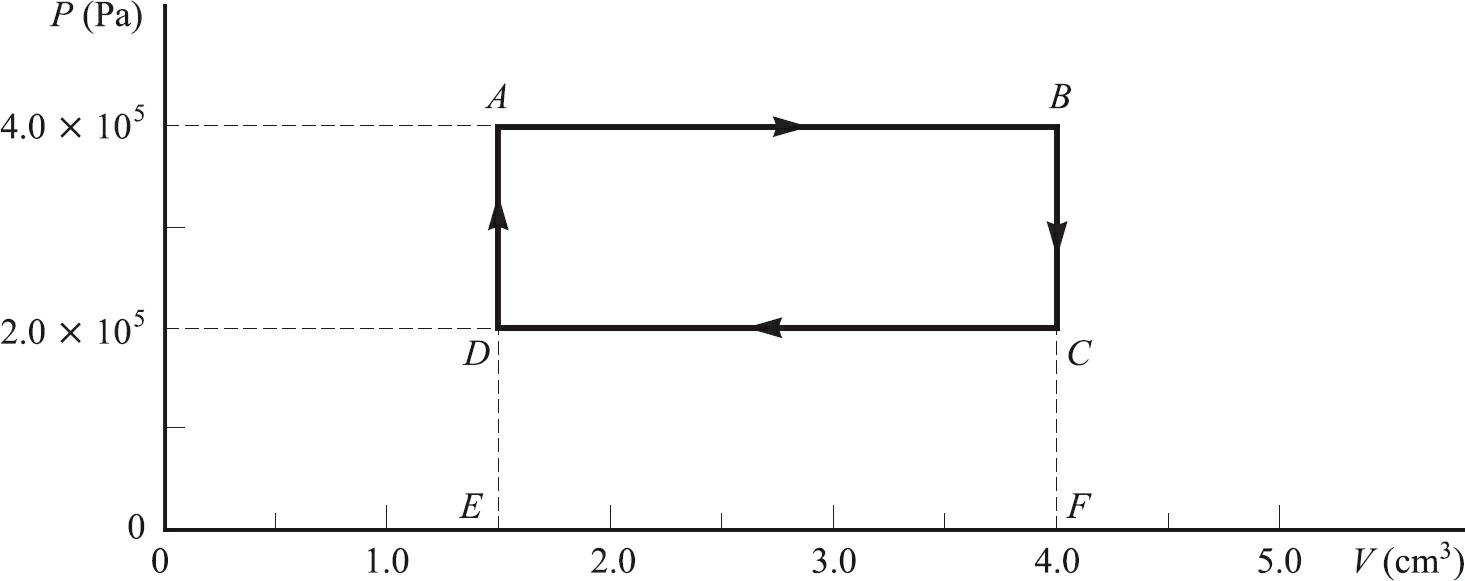
Transcribed Image Text:
P (Pa) 4.0 × 105 2.0 × 105 0 0 1.0 A D E 2.0 3.0 B C F 4.0 5.0 V (cm³)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Solution a The net work output of the gas during the cycle is equal to the area enclosed by the rect...View the full answer

Answered By

Ram Sonwane
I have qualified NET JRF exam with AIR 158 rank and has almost 3 years of teaching experience at different engineering colleges. I have great commond over ODE PDE and Calculus. I can go to the depth of topic easily and correlates them with different topics .I help the students to understand and differentiate the topic with my own tricks.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
The gas-turbine cycle shown in Fig P11.54 is used as an automotive engine. In the first turbine, the gas expands to pressure P5, just low enough for this turbine to drive the compressor. The gas is...
-
A R-12 heat pump cycle shown in Fig P6.47 has a R-12 flow rate of 0.05 kg/s with 4 kW into the compressor. The following data are given Calculate the heat transfer from the compressor, the heat...
-
The basic layout of a combined Brayton-Rankine cycle is shown in Fig. 9.49. Air enters the isentropic compressor at 10°C and 100 kPa, with a mass flux of 30 kg/s. The pressure ratio for this...
-
Why would an organization use outplacement strategies before downsizing? Outplacing employees helps former employees develop new skill sets. Outplacement strategies are a means of eliminating problem...
-
Discuss the probability versus risk trade-offs associated with alternative levels of working capital investment.
-
Susan Visscher, owner of Visschers Hardware, is negotiating with First Merchants Bank for a $50,000, one-year loan. First Merchants has offered Visscher the following alternatives. Calculate the EAR...
-
When Time, Inc., and Warner Communications merged, it represented one of the largest busi ness combinations of all time. Since then the company has reported consistent net losses despite relatively...
-
Pet Transport Company makes two pet carriers, the Cat-allac and the Dog-eriffic. They are both made of plastic with metal doors, but the Cat-allac is smaller. Information for the two products for the...
-
Nance Company estimates that its annual warranty expense is 4% of net sales. The following information relate to calendar year 2020. Net sales: Unadjusted balance of Provision for Product Warranty:...
-
How much work is done by an ideal gas in expanding isothermally from an initial volume of 3.00 liters at 20.0 atm to a final volume of 24.0 liters?
-
For nitrogen gas, c = 740 J/kg K. Assuming it to behave like an ideal gas, find its specific heat at constant pressure. (The molecular mass of nitrogen gas is 28.0 kg/kmol.)
-
Cooke borrowed money from Haddon and gave as collateral four cases of champagne and a promissory note. When Cooke failed to pay the debt, Haddon obtained a judgment for the outstanding balance. Cooke...
-
= The momentum transfer is q = ph - Ph, where p is the hadron momentum after the collision. This relationship holds for the time as well as the space components, i.e., for the 4-vectors. Thus, we...
-
When should HR be the interviewer, and when should a hiring manager or co-workers be involved? Should reference checking be done before or after the interview? Would you ask during the interview any...
-
1. Monoclean Company manufactures a single product, Glamour. The standard cost specification sheet shows the following standards for one unit of Glamour: 8 kg of material M @ $6.5 per kg $52 4 hours...
-
Fineas Co. use the Job Order Costing system to determine product costs. Before entering 2020, the company has created a production budget, with an estimated total manufacturing overhead of $...
-
Define what a market value is? What are three major principles of investing funds? How does the federal government control the money supply? An investor purchases a 10-year U.S. Treasury note and...
-
An automatic teller machine (ATM) is being installed at a branch of MetroBank. From the bank's research, it figures to indirectly benefit from offering this service. Estimates are that the bank will...
-
2. In the circuit given in Figure 2, i,(t) = 5.67cos(5t)A and v (t) = 70.71 cos(5t 60) V a) Find the equivalent load impedance. State whether the load is inductive or capacitive. b) Calculate the...
-
The dimensions of the outer conductor of a coaxial cable are b and c, where c > b. Assuming = 0 , find the magnetic energy stored per unit length in the region b < < c for a uniformly distributed...
-
The cones θ = 21¦ and θ = 159¦ are conducting surfaces and carry total currents of 40 A, as shown in Figure 8.17. The currents return on a spherical conducting...
-
Determine the energy stored per unit length in the internal magnetic field of an infinitely long, straight wire of radius a, carrying uniform current I .
-
Both answers please Problem - 7 Akimora Dairy began operations on April 1, 2015, with purchase of 250 miking cows for 18.500.000. It has completed the first month of operations and has the following...
-
need help answering questions 3. What is the deferral adjustment associated with deferred revenue? a. Dr. Deferred Revenue (liability - BS), Cr. Revenue (revenue - IS) 4. What is the deferral...
-
Hosmer Corporation issued $190,000 par value, 6%, 4-year bonds (i.e., there were 190 of $1,000 par value bonds in the issue). Interest is payable semiannually each January 1 and July 1 with the first...

Study smarter with the SolutionInn App


