A mixture of 60 mol% propylene and 40 mol% propane at a flow rate of 100 lbmol/h and at 25C and 300 psia is to
A mixture of 60 mol% propylene and 40 mol% propane at a flow rate of 100 lbmol/h and at 25°C and 300 psia is to be separated with a polyvinyltrimethylsilane polymer (see Table 14.10 for permeabilities). The membrane skin is 0.1 mm thick, and spiral-wound modules are used with a pressure of 15 psia on the permeate side. Calculate the material balance and membrane area in m2 as a function of the cut (fraction of feed permeated) for:
(a) Perfect-mixing flow pattern and
(b) Crossflow pattern.
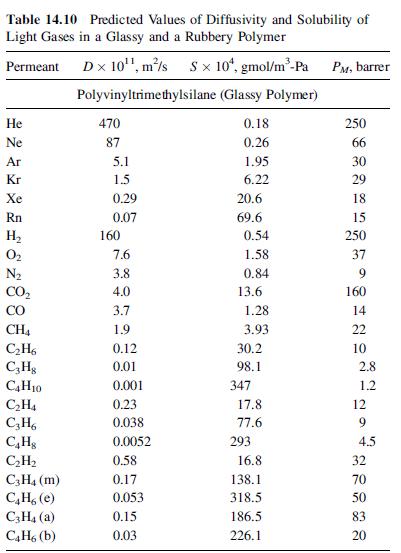
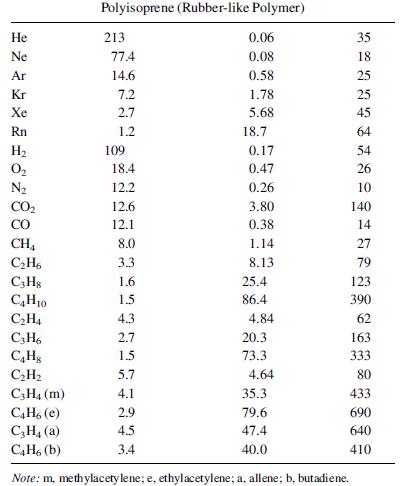
Table 14.10 Predicted Values of Diffusivity and Solubility of Light Gases in a Glassy and a Rubbery Polymer Permeant Dx 10 m/s Sx 10, gmol/m-Pa Polyvinyltrimethylsilane (Glassy Polymer) 0.18 0.26 He Ne Ar Kr Xe Rn H 0 CH4 CH6 C3H8 C4H10 CH4 C3H6 CH8 CH C3H4 (m) C4H, (e) C3H (a) C4H6 (b) 470 87 5.1 1.5 0.29 0.07 160 7.6 3.8 4.0 3.7 1.9 0.12 0.01 0.001 0.23 0.038 0.0052 0.58 0.17 0.053 0.15 0.03 1.95 6.22 20.6 69.6 0.54 1.58 0.84 13.6 1.28 3.93 30.2 98.1 347 17.8 77.6 293 16.8 138.1 318.5 186.5 226.1 PM, barrer 250 66 30 29 18 15 250 37 9 160 14 22 10 2.8 1.2 12 9 4.5 32 70 50 83 20
Step by Step Solution
3.35 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
a Perfectmixing flow pattern VfFcXfXoA The mass flow rate is Vf t... View full answer

Get step-by-step solutions from verified subject matter experts
100% Satisfaction Guaranteed-or Get a Refund!
Step: 2Unlock detailed examples and clear explanations to master concepts

Step: 3Unlock to practice, ask and learn with real-world examples

See step-by-step solutions with expert insights and AI powered tools for academic success
-
 Access 30 Million+ textbook solutions.
Access 30 Million+ textbook solutions.
-
 Ask unlimited questions from AI Tutors.
Ask unlimited questions from AI Tutors.
-
 Order free textbooks.
Order free textbooks.
-
 100% Satisfaction Guaranteed-or Get a Refund!
100% Satisfaction Guaranteed-or Get a Refund!
Claim Your Hoodie Now!

Study Smart with AI Flashcards
Access a vast library of flashcards, create your own, and experience a game-changing transformation in how you learn and retain knowledge
Explore Flashcards





