A total of 6,000 lb/h of a liquid solution of 40 wt% benzene in naphthalene at 50
Question:
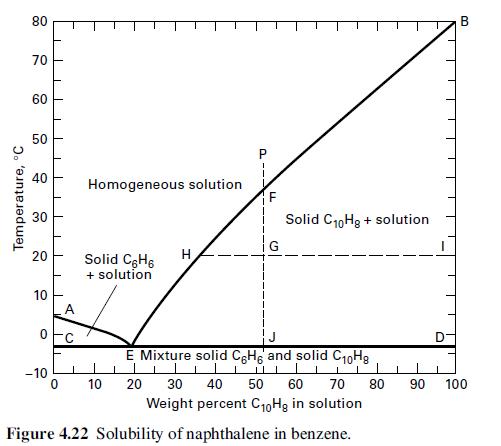
A total of 6,000 lb/h of a liquid solution of 40 wt% benzene in naphthalene at 50οC is cooled to 15οC. Use Figure 4.22 to obtain the weight of crystals and the flow rate and composition of mother liquor. Are the crystals benzene or naphthalene?
Transcribed Image Text:
Temperature, °C 80 70 60 50 40 20 10 O -10 Homogeneous solution Solid C6H6 + solution 10 H F G Solid C₁0Hg + solution B E Mixture solid CeHg and solid C10Hg ||| 1 1 20 30 40 50 60 70 80 90 100 Weight percent C10Hg in solution Figure 4.22 Solubility of naphthalene in benzene.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)
Solution The crystals are naphthalene The volume of t...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:
Students also viewed these Life Sciences questions
-
What is the probability of throwing a total of 6 points or less with three dios? n
-
A liquid containing 60 mol% toluene and 40 mol% benzene is continuously distilled in a single-equilibrium-stage unit at atmospheric pressure. What percent of benzene in the feed leaves in the vapor...
-
solution of Na2SO4. Determine the composition and flow rate of the product if the flow rate of Na2SO4 is 1275 kg/hr, and the ratio of the flow rate of the H2O to the product solution is 0.83.
-
You want to launch a business internationally, and you need to choose 3 countries1 in the Middle East, 1 in Asia, and 1 in Latin America. What are some of the components of these cultures that you...
-
Use the DemnatiRao (2004) method discussed in Exercise 23 of Chapter 9 to estimate V(tyGREG) fortyGREG defined in (11.20). Use the matrix differentiation result given in Exercise 20.
-
Sabas Company has 20,000 shares of $100 par, 1% non-cumulative preferred stock and 100,000 shares of $50 par common stock. The following amounts were distributed as dividends: Year 1: $10,000 Year 2:...
-
The random variable x has the following discrete probability distribution: LO9 x: 5 6 7 8 9 p(x:) .2 .3 .2 .1 .2 Since the values that x can assume are mutually exclusive events, the event 5x 76 is...
-
Flanders, Inc., has a $700,000, 8 percent bond issue that was issued a number of years ago at face value. There are now 10 years left on the bond issue, and the market interest rate is 16 percent....
-
I need the solution to this problem. A firm is considering two mutually exclusive prejects, X and Y, with the folloning cash flows
-
Compute FUTA & SUTA Tax: Payroll: $737,910.00 Wages in Excess of $7,000 Cap $472,120.00 SUTA Rate 2.90% Gross FUTA Rate FUTA Credit Rate Maximum Credit Allowed Compute taxes considering FUTA...
-
What is the secondary dew point? Is there also a secondary bubble point?
-
A mixture of chloroform (CHCl 3 ) and acetic acid at 18 C and 1 atm (101.3 kPa) is extracted with water to recover the acid. Fortyfive kg of 35 wt% CHCl3 and 65 wt% acid is treated with 22.75 kg of...
-
Carl Corporation acquires a business use warehouse for $200,000 on January 2, 2011. From 2011 through 2016, Carl Corporation properly deducts a total of $30,000 in depreciation. Carl incurs a net...
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
Find the derivative of each function at the given number. f(x) = x 2 3 at 0
-
How can NAFTA be beneficial to suppliers of Walmart?
-
In the 1960s, the drug thalidomide was prescribed to pregnant women to treat morning sickness. However, thalidomide caused severe limb defects in the children of some women who took the drug, and its...
-
Name the compound shown below. Is this nucleotide a component of DNA, RNA, or both? Name one other function of this compound. IN HN, E 8CH 9, N. 'N. ,N 5" -0-P-0-0-P-0-0-P-0 , 2"
-
The chemical basis of blood-group specificity resides in the carbohydrates displayed on the surface of red blood cells. Carbohydrates have the potential for great structural diversity. Indeed, the...
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...

Study smarter with the SolutionInn App


