Question: Two feedsF at 7,500 kg/h containing 50 wt% acetone and 50 wt% water, and F0 at 7,500 kg/h containing 25 wt% acetone and 75 wt%
Two feeds—F at 7,500 kg/h containing 50 wt% acetone and 50 wt% water, and F0 at 7,500 kg/h containing 25 wt% acetone and 75 wt% water—are to be extracted in a system with 5,000 kg/h of 1,1,2-trichloroethane at 25οC to give a 10 wt% acetone raffinate. Calculate the stages required and the stage to which each feed should be introduced using a right-triangle diagram. Equilibrium data are in Exercise
Data From Exercise 8.11
One thousand kg/h of a 45 wt% acetone-in-water solution is to be extracted at 25οC in a continuous, countercurrent system with pure 1,1,2-trichloroethane to obtain a raffinate containing 10 wt% acetone. Using the following equilibrium data, determine with an equilateral-triangle diagram:
(a) The minimum flow rate of solvent;
(b) The number of stages required for a solvent rate equal to 1.5 times minimum;
(c) The flow rate and composition of each stream leaving each stage.
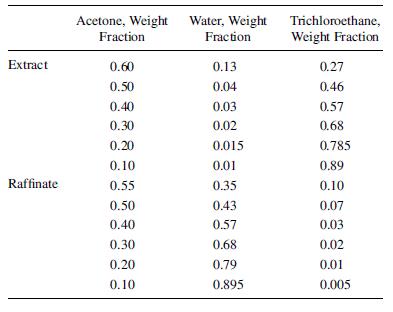
The tie-line data are:
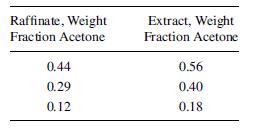
Extract Raffinate Acetone, Weight Fraction 0.60 0.50 0.40 0.30 0.20 0.10 0.55 0.50 0.40 0.30 0.20 0.10 Water, Weight Fraction 0.13 0.04 0.03 0.02 0.015 0.01 0.35 0.43 0.57 0.68 0.79 0.895 Trichloroethane, Weight Fraction 0.27 0.46 0.57 0.68 0.785 0.89 0.10 0.07 0.03 0.02 0.01 0.005
Step by Step Solution
3.46 Rating (149 Votes )
There are 3 Steps involved in it
a The minimum flow rate of solvent 15 times the minimum flow rate of solvent 30... View full answer

Get step-by-step solutions from verified subject matter experts


