Calculate the number of tetrahedral atoms per nm 3 for each zeolite framework type listed in the
Question:
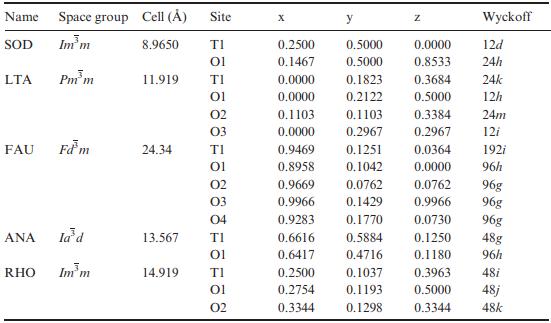
Calculate the number of tetrahedral atoms per nm3 for each zeolite framework type listed in the table below.
Transcribed Image Text:
Name Space group Cell () SOD Imm LTA FAU ANA RHO Pmm Fdm la'd Im m 8.9650 11.919 24.34 Site TI 01 14.919 TI 01 02 03 TI 01 02 03 04 13.567 TI 01 TI 01 02 X 0.2500 0.5000 0.1467 0.5000 0.0000 0.1823 0.0000 0.2122 0.1103 0.0000 0.9469 0.8958 0.9669 0.9966 0.9283 y 0.2500 0.2754 0.3344 0.1103 0.2967 0.1251 0.1042 0.0762 0.1429 0.1770 0.6616 0.5884 0.6417 0.4716 0.1037 0.1193 0.1298 Z 0.0000 0.8533 0.3684 0.5000 0.3384 0.2967 0.0364 0.0000 0.0762 0.9966 0.0730 0.1250 0.1180 0.3963 0.5000 Wyckoff 12d 24h 24k 12h 24m 12i 192i 96h 96g 96g 96g 48g 96h 48i 48j 0.3344 48k
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
See table Note cells are for the framework type such that T per nm ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Zeolite A can be described using a 11.9 primitive cubic cell with the origin at a -cage centerin Figure 14.3. From the figure, make a visual estimate of the size of a sphere that would fit inside...
-
The -cristobalite polymorph of SiO 2 has a tetragonal cell with a = 4.971 , b = 6.922 , with Si on 2a and O on 4f Wyckoff sites. The SiO bond length is 1.60 and the average OO distance within...
-
For the following two graphics, provide the specified information below for each. Inverse Demand: P= 43.75 - .00625 Q; MR = 43.75 - 0.0125 Q 25 20 15 $ per unit 10 10 5 0 MC 500 1000 1500 ATC 2000 -...
-
Use the accompanying graph of y = f(x). Does exist? If it does, what is it? lim f(x)
-
Using the Hund rules, find the magnetic moment of the ground state of the atom whose open sub shell is half-filled with five electrons.
-
The profits from a partnership are to be distributed so that Grace receives 20% more than Kajsa, and Mary Anne receives five-eighths as much as Grace. How much should each receive from a total...
-
10. Give examples of some miscellaneous provisions that might be found in a contract.
-
Refer to the Contois Carpets problem, for which the network representation is shown in Figure. Suppose that Contois has a beginning inventory of 50 yards of carpet and requires an inventory of 100...
-
FINANCIAL REPORTING INDIVIDUAL ASSIGNMENT (22 MARCH 2020) Marks: [35] NB: Please use the answer sheet provided. You may use non-programmable calculators. QUESTION 1: (15 MARKS) Answer each of the...
-
The table above contains ideal framework coordinates for different zeolites. Use a package such as vesta (http://jp-minerals.org/vesta/en/) to produce a 3D drawing of each structure. For each example...
-
Calculate the surface area of (a) A 1 g single crystal of cristobalite (density = 2.3 g/cm 3 , assume the crystal takes a spherical shape), (b) A uniform powder of spherical crystals each with a...
-
In Exercises 6473, solve each exponential equation. Where necessary, express the solution set in terms of natural or common logarithms and use a calculator to obtain a decimal approximation, correct...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
What are the major differences between the official poverty measure and the newer supplemental measure (SPM)? Provide a rationale for each of the changes made.
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
Predict the final product(s) for each sequence of reactions: (a) (b) (c) (d) Br Br. 1) Excess NaNH, 2) EECI 3) H2, Lindlar's catalyst 1) NANH, 2) Mel 3) 9-BBN 4) H202, NaOH H-CEC-H
-
When (R)-4-bromohept-2-yne is treated with H 2 in the presence of Pt, the product is optically inactive. Yet, when (R)-4-bromohex-2-yne is treated with the same conditions, the product is optically...
-
If the reaction Fe 2 N(s) + 3/2H 2 (g) 2Fe(s) + NH 3 (g) comes to equilibrium at a total pressure of 1 bar, analysis of the gas shows that at 700. and 800. K, PNH 3 /PH 2 = 2.165 and 1.083,...
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


