CeMgAl 11 O 19 :Tb 3+ is a green phosphor used in tricolor fluorescent lights. Figure 7.25
Question:
CeMgAl11O19:Tb3+ is a green phosphor used in tricolor fluorescent lights.
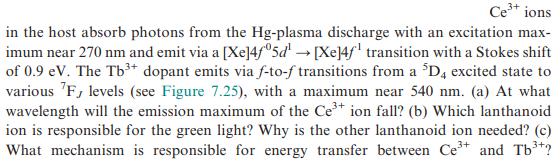
Figure 7.25
Transcribed Image Text:
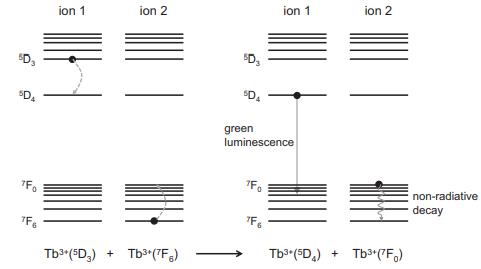
Ce+ ions in the host absorb photons from the Hg-plasma discharge with an excitation max- imum near 270 nm and emit via a [Xe]4f05d [Xe]4f transition with a Stokes shift of 0.9 eV. The Tb+ dopant emits via f-to-f transitions from a D, excited state to various F, levels (see Figure 7.25), with a maximum near 540 nm. (a) At what wavelength will the emission maximum of the Ce+ ion fall? (b) Which lanthanoid ion is responsible for the green light? Why is the other lanthanoid ion needed? (c) What mechanism is responsible for energy transfer between Ce+ and Tb*?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The absorption maximum of 270 nm corresponds to an e...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Create a conclusion to following work. Hotels form an uninterrupted narrative of a story, filling guests with emotions that define their stay. They have ceased to be considered one-dimensional places...
-
A university campus has 200 classrooms and 400 faculty offices. The classrooms are equipped with 12 fluorescent tubes, each consuming 110 W, including the electricity used by the ballasts. The...
-
A university campus has 200 classrooms and 400 faculty offices. The classrooms are equipped with 12 fluorescent tubes, each consuming 110 W, including the electricity used by the ballasts. The...
-
Robert is the owner of an automobile manufacturing company. He calls for a board meeting and tells his directors that he wants to build a car that lets the users experience power and exhilaration. He...
-
A 10-V battery supplies a constant current of 0.5amp to a resistance for 30 min (a) Determine the resistance, in ohms, (b) For the battery, determine the amount of energy transfer by work, in kJ.
-
Investigators selected an SRS of 200 high school seniors from a population of 2000 for a survey of television-viewing habits, with an overall response rate of 75%. By checking school records, they...
-
A and B are mutually exclusive events, with P1A2 = .3 and P1B2 = .4. LO5 a. Find P1A0B2. b. Are A and B independent events?
-
On January 1, 2018, National Insulation Corporation (NIC) leased equipment from United Leasing under a finance lease. Lease payments are made annually. Title does not transfer to the lessee and there...
-
Sierra Company Adjusted Trial Balance December 31 Account Title Debit Credit Cash $ 5,100 Prepaid insurance 510 Notes receivable (due in 5 years) 4,100 Buildings 20,200 Accumulated...
-
When Ce 3+ ions substitute for La 3+ in a LaCl 3 host, the lowest-energy peak in the photoluminescence excitation spectrum falls at 281 nm, whereas a similar substitution in the double-perovskite...
-
The phosphor BaMgAl 10 O 17 :Eu 2+ ,Mn 2+ , whose excitation and emission spectra are shown below, is of interest as a combined blue and green phosphor in plasma-display panels. The Eu 2+ ions absorb...
-
How does cost-based pricing differ from value-based pricing?
-
226 Payroll Accounting Chapter 7: Comprehensive Projects-Paper-Based Versions One-Month Project NOTE! Templates needed to complete these exercises, including one containing year-to-date payroll data,...
-
The Westchester Chamber of Commerce periodically sponsors public service seminars and programs Currently, promotional plans ore under way for this year's program Advertising alternatives include...
-
Mastery Problem: Activity-Based Costing WoolCorp WoolCorp buys sheep's wool from farmers. The company began operations in January of this year, and is making decisions on product offerings, pricing,...
-
The following system of linear equations is called underdetermined because there are more variables than equations. x2x 3x3 = 4 2x1x2 + 4x3 = -3 Similarly, the following system is overdetermined...
-
Write a 2000-word Reflection paper on " Country Managers Simulation by considering the following points: Countries chosen during the simulation were Argentina and Brazil: 1. Explain why you did what...
-
The Chemco Company manufactures car tires that last distances that are normally distributed with a mean of 57,300 km and a standard deviation of 6875 km. a. If a tire is randomly selected, what is...
-
Consider the reaction of acetic acid in water CH 3 CO 2 H(aq) + H 2 O(l) CH3CO 22 (aq) + H 3 O + (aq) where Ka 5 1.8 3 1025. a. Which two bases are competing for the proton? b. Which is the stronger...
-
A sample containing 2.50 mol of an ideal gas at 325 K is expanded from an initial volume of 10.5 L to a final volume of 60.0 L. Calculate G and A for this process for a. An isothermal reversible path...
-
Why does the liquidgas coexistence curve in a PT phase diagram end at the critical point?
-
An ideal gas is expanded reversibly and isothermally. Decide which of q, w, U, and H is positive, negative, or zero.
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


