For each of the following phosphors, identify the type of electronic transition responsible for luminescence and predict
Question:
For each of the following phosphors, identify the type of electronic transition responsible for luminescence and predict whether it will show weak, moderate, or strong electron–phonon coupling:
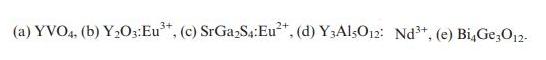
Transcribed Image Text:
(a) YVO4. (b) YO3:Eu*, (c) SrGaS4:Eu, (d) Y3AlsO12: Nd+, (e) BiG3012.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a VO 4 3 chargetransfer transition with strong electronphonon ...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
"I'm not sure we should lay out $300,000 for that automated welding machine," said Jim Alder, president of the Superior Equipment Company. "That's a lot of money, and it would cost us $84,000 for...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Identify whether each of the following reagents would be a strong nucleophile or a weak nucleophile, and also indicate whether it would be a strong base or a weak base: a.
-
Elastic bands are attached to tuning fork 1 ( which was 2 5 6 Hz ) to reduce its frequency. It is sounded again with tuning fork 2 ( 2 5 5 Hz ) , making 1 2 beats in 6 . 0 s . What is the new...
-
The belt sander shown in Fig.P2.35 has a belt speed of 1500 ft/min. the coefficient of friction between the sander and a plywood surface being finished is 0.2. If the downward (normal) force on the...
-
Show that when the optimal allocation is used, the variance of (2) str for two-phase sampling with stratification is given by (12.18). Discuss.
-
Why is Bayess rule unnecessary for finding P1B0A2 if events A and B are mutually exclusive? LO5
-
Merchandise is sold on account to a customer for $12,500, terms FOB shipping point, 1/10, n/30. The seller paid the freight of $250. Determine the following: (a) Amount of the sale, (b) Amount...
-
On January 1, 2021, Blossom Corp. had 2,781,000 shares of common stock issued and outstanding. During 2021, it had the following transactions that related to common stock. Mar. 1 Issued 273,000...
-
As the difference Q between the equilibrium bond distances of the ground and excited states increases, would you expect the following phosphor characteristics to increase, decrease, or be unaffected:...
-
The Pr 3+ ion has 91 microstates that can be grouped into six terms before spinorbit coupling is included: 1 D, 3 F, 1 G, 3 H, 3 P, 1 S. (a) What is the electron configuration of Pr 3+ ? (b) What are...
-
Jans All You Can Eat Restaurant charges $8.95 per customer to eat at the restaurant. Restaurant management finds that its expense per customer, based on how much the customer eats and the expense of...
-
Question TARIMAX MASTO Copper Explorations recently acquired the rights to mine a new site. Machinery, equipment and a truck were purchased to begin the mining operations at the site. Details of the...
-
Exercise 6 - 6 ( Algo ) The Town of Weston has a Water Utility Fund with the following trial balance as of July 1 , 2 0 2 3 , the first day of the fiscal year: During the year ended June 3 0 , 2 0 2...
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
What is the correct answer to this? SQL QUESTION Sales Data for All Customers and Products Write a query that will return sales details of all customers and products. The query should return all...
-
Below are the jersey numbers of 11 players randomly selected from a football team. Find the range, variance, and standard deviation for the given sample data. What do the results tell us? 84 18 34 3...
-
Suppose that when a grocery store has 3 cashiers working during its afternoon rush period, a customer waits 8 minutes on average to be served. If there are 2 cashiers working, what is the probability...
-
Is it ethical to provide safety training in English to immigrant workers who speak little English, in order to reduce costs?
-
A 0.1429 g sample of sucrose, C 12 H 22 O 11 , is burned in a bomb calorimeter. In order to produce the same temperature rise in the calorimeter as the reaction, 2353 J must be expended. a. Calculate...
-
Identify whether you would use dilute sulfuric acid or concentrated sulfuric acid to achieve each of the following transformations. In each case, explain your choice. a. b. [H,SO,] - . [H,SO,] + H20
-
Under anaerobic conditions, glucose is broken down in muscle tissue to form lactic acid according to the reaction: C 6 H 12 O 6 (s) 2CH 3 CHOHCOOH(s). Thermodynamic data at T = 298 K for glucose and...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


