Question: Into the figure below, sketch the ordered d z 2 orbitals of Mn 3+ such that their orientation explains the magnetic moments in this La
Into the figure below, sketch the ordered dz2 orbitals of Mn3+ such that their orientation explains the magnetic moments in this La1/3Ca2/3MnO3 layer. Use the principle that follows from the magnetic order of La0.5Ca0.5MnO3 in Figure 11.9.
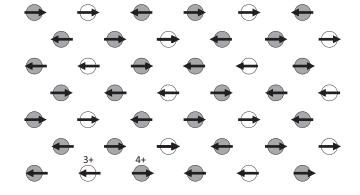
Figure 11.9
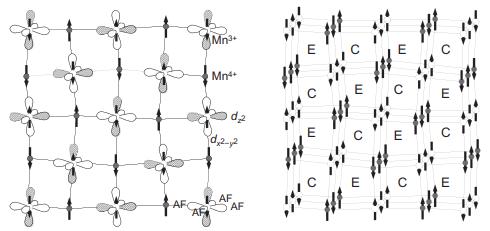
Step by Step Solution
3.34 Rating (160 Votes )
There are 3 Steps involved in it
To identify ferromagnetic couplings select one spin dire... View full answer

Get step-by-step solutions from verified subject matter experts


