Mass susceptibility of Sr 2 MnMoO 6 (molar mass 422.114 g/mol) was measured as a function of
Question:
Mass susceptibility of Sr2MnMoO6 (molar mass 422.114 g/mol) was measured as a function of temperature, from which the Curie constant, Cm, was obtained by leastsquares fitting as Cm = 1.306×10−4m3 K/kg. Determine the oxidation states of Mn and Mo. Select an expression for the Curie constant C in Equation (9.19). Replace μeff in it with μeff/μB so that μeff is now in Bohr magnetons. Assuming two magnetic atoms performula, Mn and Mo, calculate the number N of these atomsin one kilogram of the sample.
Equation (9.19)
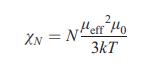
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





