Question: Why are there no examples of fluorides with the CdI 2 or CdCl 2 structures (Figure 1.28)? Figure 1.28 Cdl-type stacking Plane of octahedral holes
Why are there no examples of fluorides with the CdI2 or CdCl2 structures (Figure 1.28)?
Figure 1.28
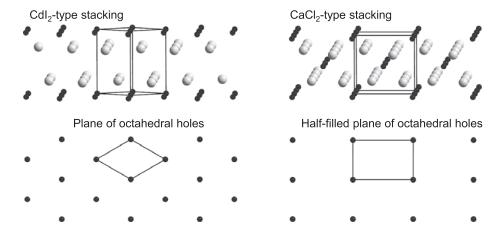
Cdl-type stacking Plane of octahedral holes CaCl-type stacking Half-filled plane of octahedral holes
Step by Step Solution
3.35 Rating (164 Votes )
There are 3 Steps involved in it
CdI 2 and CdCl 2 form layered structures with cati... View full answer

Get step-by-step solutions from verified subject matter experts


