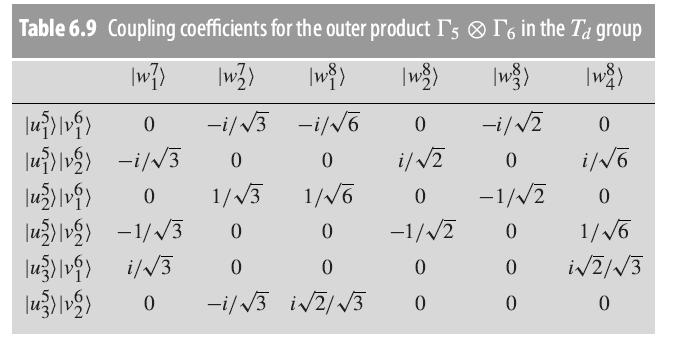
Question: Verify, using the coupling coefficients for the 6 5 product states in Table 6.9, that the interaction (6.6.5) acting on the product
Verify, using the coupling coefficients for the Γ6 ⊗ Γ5 product states in Table 6.9, that the interaction (6.6.5) acting on the product states gives the same energy shift for both Γ7 states, and a common energy shift for the four Γ8 states.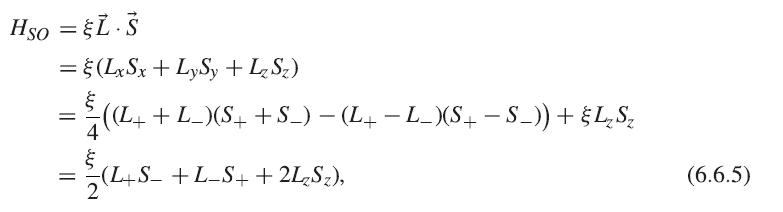
Hso = L. = (LxSx + LySy + Lz Sz) ((L+ + L_)(S+ + S_) (L+ L_)(S+ S_)) + LS - =(L+S_+L_S+ + 2LzS), = || = (6.6.5)
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
In order to calculate the result of the action of the operator L5 we compute the effect of L L Lz on u lu Ju and the effect of S S S on v and v Simila... View full answer

Get step-by-step solutions from verified subject matter experts


