As part of a study on the rate of combustion of artificial graphite in humid air flow,
Question:
As part of a study on the rate of combustion of artificial graphite in humid air flow, researchers conducted an experiment to investigate oxygen diffusivity through a water vapor mixture. A 3 × 9 factorial experiment was conducted with mole fraction of water (H2O) at three levels and temperature of the nitrogen–water mixture at nine levels. The data are shown in the table.
a. Explain why the traditional analysis of variance (using the ANOVA formulas) is inappropriate for the analysis of these data.
b. Plot the data to determine if a first- or second-order model for mean oxygen diffusivity, E(y), is more appropriate.
c. Write an interaction model relating mean oxygen diffusivity, E(y), to temperature x1 (in hundreds) and mole fraction x2 (in thousandths).
d. Suppose that temperature and mole fraction do not interact. What does this imply about the relationship between E(y) and x1 and x2?
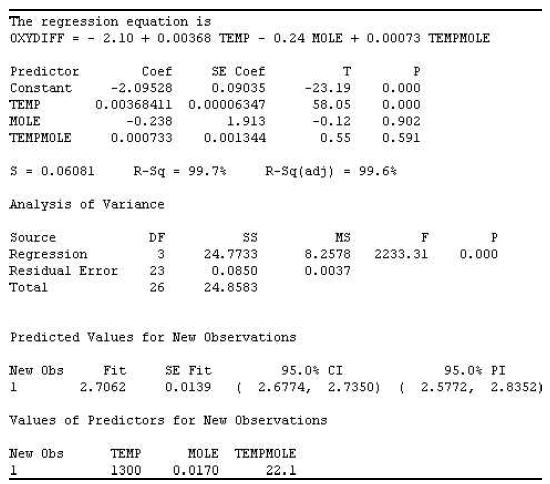
e. Do the data provide sufficient information to indicate that temperature and mole fraction of H2O interact? Use the accompanying MINITAB printout to conduct the test using α = .05.
f. Give the least-squares prediction equation for E( y).
g. Substitute into the prediction equation to predict the mean diffusivity when the temperature of the process is 1,300 K and the mole fraction of water is .017.
h. Locate the 95% confidence interval for mean diffusivity when the temperature of the process is 1,300 K and the mole fraction of water is .017 on the MINITAB printout. Interpret the result.
Step by Step Answer:

Statistics For Engineering And The Sciences
ISBN: 9781498728850
6th Edition
Authors: William M. Mendenhall, Terry L. Sincich




