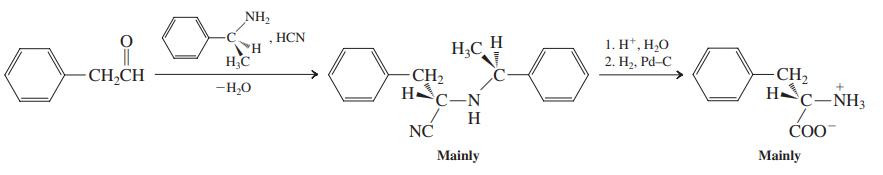
(a) Illustrate the Strecker synthesis of phenylalanine. Is the product chiral? Does it exhibit optical activity? (b)...
Question:
(a) Illustrate the Strecker synthesis of phenylalanine. Is the product chiral? Does it exhibit optical activity?
(b) It has been found that replacement of NH3 by an optically active amine in the Strecker synthesis of phenylalanine leads to an excess of one enantiomer of the product. Assign the R or S confi guration to each stereocenter in the following structures, and explain why the use of a chiral amine causes preferential formation of one stereoisomer of the final product.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





