A synthesis of the sesquiterpene farnesol requires the conversion of a dichloro compound into an alkynol, as
Question:
A synthesis of the sesquiterpene farnesol requires the conversion of a dichloro compound into an alkynol, as shown below. Suggest a way of achieving this transformation.
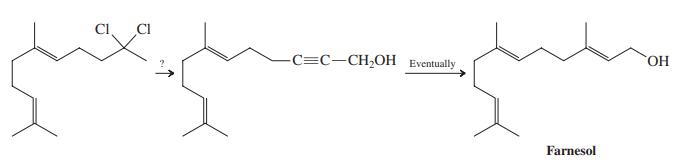
Transcribed Image Text:
CI. CI -C=C-CH;OH Eventually HO, Farnesol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
First we can use a bulky base such as Potassium tertbutoxide KOCH33 to ...View the full answer

Answered By

Akop Yepremyan
My aim would be to adequately walk through a given problem and find a solution through logic, rather than memorizing each question. With my strong background in tutoring and research as a PhD chemist from Texas Christian University (TCU) I will provide both theoretical and practical knowledge for your success.
I have been studying, teaching and researching in the field of chemistry for over 15 years. I have mentored and conducted multiple review sessions for students. Because of my extensive laboratory experience, I allow the student the visualize a given problem, thus making them much more enjoyable and easier to solve.
I am happy to teach you the fundamentals of chemistry in a way that will be very enjoyable for you and you are not counting the minutes to stop studying. Chemistry takes time to learn, I will make sure you push through the barrier that makes chemistry a difficult subject, and reach a level where you are very comfortable with solving any problem that is presented to you. I am available to help anytime and will respond to your need promptly.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Synthesis of the sesquiterpene bergamotene proceeds from the alcohol shown here. Suggest a sequence to complete the synthesis. HOH,C CH3 CH3 Bergamotene
-
Using benzene, acetic anhydride and 1-propanethiol as the source of all the carbon atoms, along with any necessary inorganic reagents, outline a synthesis of the compound shown.
-
Using benzene, acetic anhydride, and 1-propanethiol as the source of all the carbon atoms, along with any necessary inorganic reagents, outline a synthesis of the compound shown. 0 lonscn.oua CCH...
-
Estimates have been presented to Holly Farms, which is considering two environmental chambers for a project that will detail laboratory confirmations of on-line bacteria tests in chicken meat for the...
-
Build a WBS for one of the chapter case study projects other than asset management; either customer relationship management or Collection Management. The Project should use an iterative approach and...
-
Wapato Corporation purchased a new piece of equipment at the beginning of Year 1 for $1,000,000. The expected life of the asset is 20 years with no residual value. The company uses straight-line...
-
I:5-37 Basis of Property Converted from Personal Use. Irene owns a truck costing $15,000 and used for personal activities. The truck has a $9,600 FMV when it is transferred to her business, which is...
-
Tate Inc. has beginning work in process $26,000, direct materials used $240,000, direct labor $220,000, total manufacturing overhead $180,000, and ending work in process $32,000. What are the total...
-
A bank made a 3-month $100 million loan at 6% funded by a 6-month deposit at 2%.To protect against interest rates when rolling over the loan in three months, the bank decides to hedge using a forward...
-
The force F is applied to the handle of the box wrench. Determine the component of the moment of this force about the z axis which is effective in loosening the bolt. Given: a = 3 in b = 8 in c = 2...
-
Formulate a plausible mechanism for the hydration of ethyne in the presence of mercuric chloride. MR 3 H IH 1H 1 H (CH3)4Si 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 300-MHz 'H NMR...
-
Your team is studying the problem of an intramolecular ring closure of enediyne systems important in the total synthesis of dynemicin A, which exhibits potent antitumor activity. One research group...
-
Which of the following statements is true regarding reporting net assets in the governmentwide financial statements? a. Net assets are reported for both governmental activities and business-type...
-
Describe A demographic profile of the population and community that will be served through the reinvented Human Service program. The description must include all eligibility requirements (i.e.,...
-
You work for a major financial institution. Your branch handles customer calls from a wide variety of individuals. Recently, you've noticed an increase in calls from individuals from African...
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
Write a program RecoverSignal that will read the binary file written by StoreSignal, as described in the previous exercise. Display the integer values that the data represents on the screen.
-
Eleni Cabinet Company sold 2,200 cabinets during 2011 at $160 per cabinet. Its beginning inventory on January 1 was 130 cabinets at $56. Purchases made during the year were as follows: February . 225...
-
The CIO2 molecule (which belongs to the group C, 2v) was trapped in a solid. Its ground state is known to be BJ Light polarized parallel to the y-axis (parallel to the 00 separation) excited the...
-
What states of (a) Anthracene, (b) Coronene (24) may be reached by electric dipole transitions from their (totally symmetrical) ground states? 24 Coronene
-
Determine whether the integral over /J and /, in Exercise 12.15a is zero over a symmetrical range about e = 0 in the group C3v
-
Morning Dove Company manufactures one model of birdbath, which is very popular. Morning Dove sells all units it produces each month. The relevant range is 02,300 units, and monthly production costs...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
The following unadjusted trial balance is prepared at fiscal year-end for Nelson Company. Nelson Company uses a perpetual inventory system. It categorizes the following accounts as selling expenses:...

Study smarter with the SolutionInn App


