Attempted CrO 3 oxidation of 1,4-butanediol to butanedioic acid results in signifi cant yields of g-butyrolactone. Explain
Question:
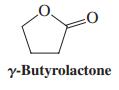
Attempted CrO3 oxidation of 1,4-butanediol to butanedioic acid results in signifi cant yields of “g-butyrolactone.” Explain mechanistically.
Transcribed Image Text:
:0 y-Butyrolactone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
HO CH HOCH CH 0CH CH CH H CO...View the full answer

Answered By

DIPAK SHETE
Hello guys, I dipak master in Chemistry. I have three years teaching experience in science and six months experience in online tutor (Q&A Expert). I able to solve any doubt in chemical sciences and mathematics also.
I have solved several problems of peoples regarding chemical sciences. I totally believe on knowledge increases
By sharing with others. And I always search someone with I can share my knowledge.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Explain the outcome of the following transformations mechanistically. (a) (b) (c) CHCH OH HSCH CH2BrNaOH DMF BrCH-CH,CH-CH,CHBr + NaOH- Excess BrCH CH CH CH CH,Br+ NH, CHCHO Excess
-
When 1,4- and 1,5-dicarboxylic acids, such as butanedioic (succinic) acid (Section 19-8), are treated with SOCl2 or PBr3 in attempted preparations of the diacyl halides, the corresponding cyclic...
-
Oxidation of an aldehyde yields a carboxylic acid: Draw the structures for the products of the following oxidation reactions. a. b. c. [ox] propanal 2,3-dimethylpentanal ox] 3-ethylbenzaldehyde>
-
If you were in charge of B&D, indicate the possible decisions you might take as regards the four Ps and the points you would want to keep in mind as you go about the task of marketing for...
-
Write down three industries in which you suspect that strategic trade policy is a tool that governments could potentially use, and three in which you suspect that it is not. Explain your reasoning in...
-
Draw a representation of an initially empty vector A after performing the following sequence of operations: insert(0,4), insert(0,3), insert(0,2), insert(2,1), insert(1,5), insert(1,6), insert(3,7),...
-
(Entries for Zero-Interest-Bearing Note) On December 31, 2007, Jose Luis Company acquired a computer from Cuevas Corporation by issuing a $400,000 zero-interest-bearing note, payable in full on...
-
The following accounts were taken from the financial statements of Orville Company. ______ Interest revenue ______ Common stock ______ Utilities payable ______ Accumulated depreciationequipment...
-
Describe how foreign currency forward contracts and foreign currency options can be used to hedge foreign exchange risk.
-
1. Choose one of the classes and create a set of invariants for attributes and relationships and add them to the CRC card for the class. 2. Choose one of the methods in the class that you chose and...
-
How would you expect the acidity of acetamide to compare with that of acetic acid? With that of acetone? Which protons in acetamide are the most acidic? Where would you expect acetamide to be...
-
Following the general mechanistic scheme, write detailed mechanisms for each of the following substitution reactions. (These transformations are part of Chapter 20, but try to solve the problem...
-
In which rock types are oil and gas found?
-
check if each transaction is placed in the right place in each of the reports below and if there are any other mistakes in the different accounts after the first image which is a description of the...
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Show how shared memory can be used to implement message passing. Specifically, choose a set of message-passing operations (e.g., no-wait send and explicit message receipt) and show how to implement...
-
A supermarket chain is interested in exploring the relationship between the sales of its store-brand canned vegetables (y), the amount spent on promotion of the vegetables in local newspapers (x1)...
-
Name these compounds: CH3 a) CHCHCHCHCHCH, b) NHCH, NH
-
Draw structures for these compounds: (a) Diethyl ammonium bromide (b) N-Methyl-3-(1-methylpropyl)-2-octanamine
-
Name thesecompounds: CH3 CH3 T a) CHCH,CH_CHCHCH,CH, CHCH3 b) CHCH=CHCHCHCH3 CI c) CH,CH,CHC=CH d) e) f) g) OH CH3 H
-
What is meant by "presentation of financial statement information in common-size amounts rather than dollar amounts?" Why is this type of presentation sometimes more meaningful than use of actual...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Conroy Company manufactures two products B 1 0 0 and A 2 0 0 . The company provided the...
-
Based on the following information, what amount would be subtracted from the bank balance side of a checking account reconciliation? Service charge $63, Outstanding checks $350, Interest $23, Deposit...

Study smarter with the SolutionInn App


