Attempted Hofmann elimination of an amine containing a hydroxy group on the b-carbon gives an oxacyclopropane product
Question:
Attempted Hofmann elimination of an amine containing a hydroxy group on the b-carbon gives an oxacyclopropane product instead of an alkene.
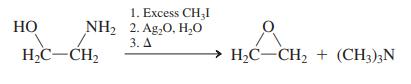
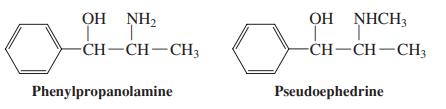
(a) Propose a sensible mechanism for this transformation. (b) Pseudoephedrine (see Problem 42) and ephedrine are closely related, naturally occurring compounds, as the similar names imply. In fact, they are stereoisomers. From the results of the following reactions, deduce the precise stereochemistries of ephedrine and pseudoephedrine.
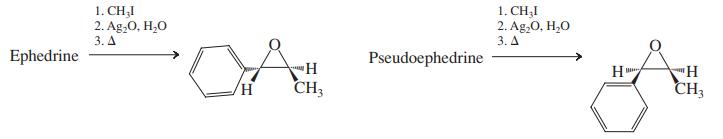
Problem 42
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





