Explain the following reaction sequence. 1. Pd(OCCH,), R,P, K.CO, COCH,CH; Br 2. H,C=CH-COCH.CH,
Question:
Explain the following reaction sequence.
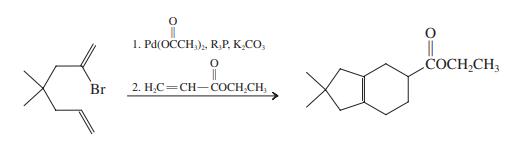
Transcribed Image Text:
1. Pd(OCCH,), R,P, K.CO, COCH,CH; Br 2. H,C=CH-COCH.CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
Mechanism Step involved Oxidative addition of pd in ...View the full answer

Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
The following reaction sequence represents an elegant method of synthesis of 2-deoxy-d-ribose, IV, published by D. C. C. Smith in 1955: (a) What are the structures of II and III? (b) Propose a...
-
In an attempt to make 1-chloro-l-cyclobutylpentane, the following reaction sequence was employed. The actual product isolated, however, was not the desired molecule but an isomer of it. Suggest a...
-
The following reaction sequence gives rise to two isomeric products. What are they? Explain the mechanism of their formation. OH 1. Mg 2. D.O `CH3
-
Refer to Exercise. a. Are the required conditions satisfied? b. Is multicollinearity a problem? If so, explain the consequences.
-
1. Describe how you would convince Reid and Mark that all projects are not alike. Discuss how IT projects are different from projects in other disciplines and why this matters. Finally, describe how...
-
Compute u in Prob. 5 for t = 0.1 and x = 0.1, 0.2, , 0.9, using the formula in Prob. 8, and compare the values. Data from Prob. 8 Compute approximate values in Prob. 7, using a finer grid (h = 0.1,...
-
What examples can individual members of the group cite of self-discipline, team discipline and managerial discipline? L01
-
Brendan Coleman created and marketed Clinex, a software billing program. Later, Retina Consultants, P. C., a medical practice, hired Coleman as a software engineer. Together, they modified the Clinex...
-
What net tax basis in MCI's assets would GTE take?
-
Refer back to the beginning of this chapter to the excerpt from a Los Angeles Times article about Reed Slatkin's fraud. The article insinuates that the FBI and IRS's raiding of Slatkin's office...
-
Which of the reactions shown below will occur under the influence of heat? Light? (a) (b) (c) H H
-
Give abbreviated structures of each of the following compounds: (a) (E)-1,4-poly-2-methyl-1,3- butadiene [(E)-1,4-polyisoprene]; (b) 1,2-poly-2-methyl-1,3-butadiene (1,2-polyisoprene); (c) 3,4-...
-
Which components of the marketing information system do Qualtricss tools facilitate? In todays environment of data proliferation, companies dont need more datathey need better data. Qualtrics...
-
a b Solve a) (725.25)10=(?)2=(?)16 b) (111100111110001)2= (?) 8 = (?) 16 Build the equation Y=AB+ CD + E to realize using a) NAND Gates b) NOR Gates Construct and describe Full Adder with neat logic...
-
With such a high base rate, you are confident about the chance of hiring and have posted the job ad based on a prior job analysis. Listed below are the final applicants and their profile of four key...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a perpetual inventory system. Determine the cost assigned to the ending inventory using FIFO. 1 Date...
-
A company may go through organizational change at various stages in its life cycle for a variety of reasons. Reasons can include a change in ownership as well as a change in the competitive...
-
6 (a) Below is a diagram of a rotating disc viscometer (FIGURE 4). Explain its operations and limitations as to use. If, in a similar works situation, it is necessary to make measurements on a...
-
Create an application that will keep track of several groups of strings. Each string will be a member of exactly one group. We would like to be able to see whether two strings are in the same group...
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
Consider a system of distinguishable particles having only three no degenerate energy levels separated by an energy which is equal to the value of kTat 25.0 K. Calculate (a) The ratio of populations...
-
At what temperature would the population of the first excited rotational level ofHCl are lie times its population of the ground state?
-
Calculate the standard molar entropy of xenon gas at (a) 100 K, (b) 298.15 K.
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
If Total Assets are $2,100 and Total Equity is $1,600 then what is the value of Total Liabilities?
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...

Study smarter with the SolutionInn App


