Fischers solution to the problem of sugar structures was actually much more difficult to achieve experimentally than
Question:
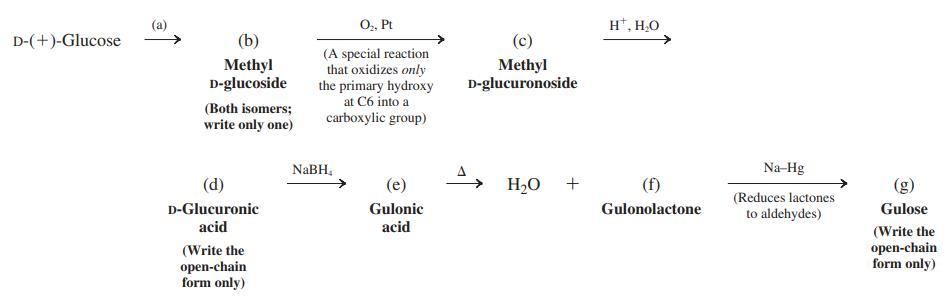
Fischer’s solution to the problem of sugar structures was actually much more difficult to achieve experimentally than implies. For one thing, the only sugars that he could readily obtain from natural sources were glucose, mannose, and arabinose. (Erythrose and threose were, in fact, not then available at all, either naturally or synthetically.) His ingenious solution required a way to exchange the functionalities at C1 and C6 of glucose and mannose in order to make the critical distinction described at the end of the section. (Of course, had gulose existed in nature, all this effort would have been unnecessary, but Fischer wasn’t so lucky.) Fischer’s plan led to unexpected diffi culties, because at a key stage he got a troublesome mixture of products. Nowadays we solve the problem in the manner shown below. Fill in the missing reagents and structures (a) through (g). Use Fischer projections for all structures. Follow the instructions and hints in parentheses.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





