Indicate which reagent or combination of reagents is best suited for each of the following reactions. (a)
Question:
Indicate which reagent or combination of reagents is best suited for each of the following reactions.
(a)
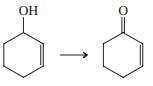
(b)
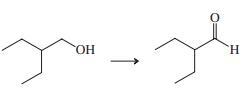
(c)
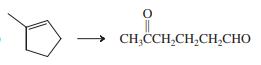
(d)
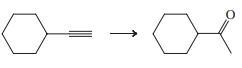
(e)
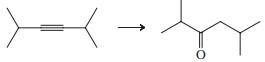
(f)
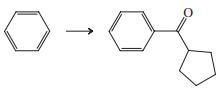
Transcribed Image Text:
OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
The given transformation can be done as a MnO 2 is usually used in selective oxidation ...View the full answer

Answered By

Shiva Prakash Singh Kushwaha
I am graduate from one of the top universities of india. I qualified GATE, CSIR - NET (JRF), TIFR National level examination . I have potential to simplifying the complicate topics related subject. Along with this i have 3 years working experience that how to deal with students that students are easily understand their mistakes. Currently i am taking a teacher training.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the product of each of the following reactions: a. b. c. d. e. f. g. h. hv CH3 H3C CH3 A DHHD H3C
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. HCI CH CH2CH CHCH2OH excess catalytic CCH CH3 NH2NH2 CH2CH3 NH2NH 1. NaBH4 0 HCI excess CH,CH,CH,COCH CHs 2. H3o 1. LIAIH...
-
Outline reasonable mechanisms for each of the following reactions: (a) (b) (c) (d) (e) (f) 0 CH2CH CH2CH2Br KOCICH3 benzene (76%) heat (CH3)2C-CHCH-CH-CCH, + CH3 (CH3hC-CHCH-CH-C-CHCH (96%) CH 0...
-
Harvold Company's quality cost report is to be based on the following data: Test and inspection of incoming materials. $71,000 Supplies used in testing and inspection . Re-entering data because of...
-
What role did societal values play in the Court's decision in this case?
-
Substituting u(r) with r as in Prob. 9 into u xx + u yy + u zz = 0, verify that u" + 2u'/r = 0, in agreement with (7). Data from Prob. 9 Show that the only solution of Laplaces equation depending...
-
1 What examples are there of managers influencing others?
-
The inventory of Wei Company on December 31, 2014, consists of the following items. aPart No. 21 is obsolete and has a realizable value of $0.20 each as scrap. Instructions (a) Determine the...
-
Table Fields Table Tell me what you want to do New Totals Calibri 11 abc Spelling Sbc Replace Go To Select Save X Delete Refresh All Find Av , - More Records Find Text Formatting File Home Create...
-
You are advising a lender group and the debtor FA provides the following summary 13-week cash flow information. Calculate the implied EBITDA for the period Receipts Payroll AP disbursements Rent...
-
Reaction review. Without consulting the Reaction Road Map on pp. 816 817, suggest reagents to convert each of the starting materials below into 3-hexanone. (a) (b) (c) (d) (e) (f) (g) (h) page 816-817
-
Write the expected products of ozonolysis (followed by mild reductione.g., by Zn) of each of the following molecules. (a) CH 3 CH 2 CH 2 CH=CH 2 (b) (c) (d)
-
The conversion time of an ADC is found to be 7.5 s. The ADC is set to convert repeatedly, with no other programming requirements. What is the maximum frequency signal it can digitize?
-
8. Look at the image to the right. Using the Law of Force and Acceleration, predict how acceleration would change if you changed the mass of the boy. 9. Using the same picture from #8, discuss how...
-
Time (s) Velocity (cm/s or m/s) Uncertainty 0.100 -145 cm/s or 0.145 m/s +/- 0.089 m/s 0.200 -266 cm/s or 0.266 m/s +/- 0.010 m/s 0.300 -359 cm/s or 0.359 m/s +/- 0.0201 m/s 0.400 -451 cm/s or 0.451...
-
Using Technology to Generate Normal Quantile Plots. In Exercises 13-16, use the data from the indicated exercise in this section. Use software (such as Statdisk, Minitab, Excel, or StatCrunch) or a...
-
Use your understanding of work and power to answer the following questions. 1. Two physics students, Will N. Andable and Ben Pumpiniron, are in the weightlifting room. Will lifts the 100-pound...
-
Problem 2. Consider the following chemical reaction. 2H2 + O2 = 2HO Gibbs Duhem equation states that SdT - Vdp+ Nidi=0. Apply this equation for the above reaction and determine the equilibrium...
-
The following information was drawn from the accounting records of Wyckoff Company as of December 31, Year 2, before the temporary accounts had been closed. The Cash balance was $3,600, and Notes...
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
Calculate the formal charges on all of the atoms except hydrogen's, in these compounds: a) H-N-N=N: c) H-C-N=N: H e) H H-=C-H b) H-N-N-N: HO: H-C-C-C 1 H d) f) H H-B-H T H
-
Explain which of the two following structures would be more stable. Explain whether they represent isomers or are resonance structures. HIN: H N-H H :0: N-H HIN
-
Draw a Lewis structure for carbon monoxide (CO). Calculate the formal charges on the atoms and comment on the stability of this compound.
-
Assume J.T. Traverse uses of 1 percent of revenue to estimate its bad debt expense for the year. Prepare the adjusting journal entry required at February 28 for recording Bad Debt Expense. Assume...
-
Cellular Access Inc., is a cellular telephone service provider that reported net operating profit after tax (NOPAT) of $ 250 million for the most recent fiscal year. The firm had depreciation...
-
Information for two alternative projects involving machinery investments follow. The accounting rate of return for Project 1 is: Year Project 1 Project 2 Initial investment $ (410,000) $ (350,000)...

Study smarter with the SolutionInn App


