The 1 H NMR spectrum of naphthalene shows two multiplets (Figure 15-16). The upfield absorption ( =
Question:
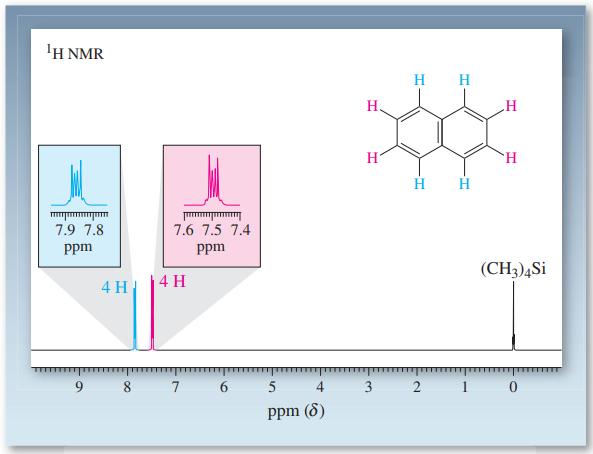
The 1H NMR spectrum of naphthalene shows two multiplets (Figure 15-16). The upfield absorption (δ = 7.49 ppm) is due to the hydrogens at C2, C3, C6, and C7, and the downfield multiplet (δ = 7.86 ppm) is due to the hydrogens at C1, C4, C5, and C8. Explain why one set of hydrogens is deshielded more than the other.
Figure 15-16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





