The benzene rings of many compounds in nature are prepared by a biosynthetic pathway similar to that
Question:
The benzene rings of many compounds in nature are prepared by a biosynthetic pathway similar to that operating in fatty acid synthesis. Acetyl units are coupled, but the ketone functions are not reduced. The result is a polyketide thiolester, which forms rings by intramolecular aldol condensation.
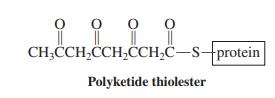
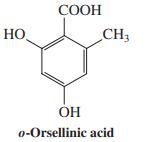
o-Orsellinic acid [for structure, see Problem 27(g)] is a derivative of salicylic acid and is prepared biosynthetically from the polyketide thiolester shown. Explain how this transformation might take place. Hydrolysis of the thiolester to give the free carboxylic acid is the last step.
Data From Problem 27 (g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





