Treatment of cyclopentane-1,3-dione with iodomethane in the presence of base leads mainly to a mixture of three
Question:
Treatment of cyclopentane-1,3-dione with iodomethane in the presence of base leads mainly to a mixture of three products.
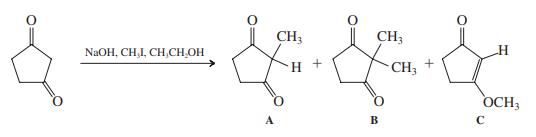
(a) Give a mechanistic description of how these three products are formed.
(b) Reaction of product C with a cuprate reagent results in loss of the methoxy group. For example,
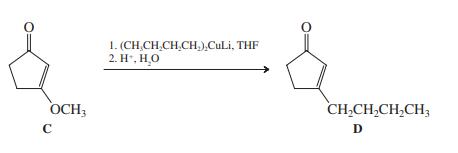
Suggest a mechanism for this reaction, which is another synthetic route to enones substituted at the β-carbon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





