What are the products of the following reaction? (a) C 6 H 5 I + CH 3
Question:
What are the products of the following reaction?
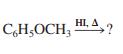
(a) C6H5I + CH3OH
(b) C6H5OH + CH3I
(c) C6H5I + CH3I
(d) 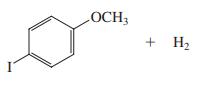
Transcribed Image Text:
CH;OCH, HL A, HI. A, ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
What are the products of the reactions of dicyclohexylethyne with the reagents in Problem 44? (a) D 2 , Pd CaCO 3 , Pb(O 2 CCH 3 ) 2 , quinoline; (b) Na, ND 3 ; (c) 1 equivalent HI; (d) 2...
-
What are the products of each of the following reactions? Your answer should account for all the amino acid residues in the starting peptides. (a) Reaction of Leu-Gly-Ser with 1-fluoro-2,...
-
What are the products of each of the following reactions? Your answer should account for all the amino acid residues in the starting peptides. (a) Reaction of Leu-Gly-Ser with...
-
20 -101 10 in- laminate substrate Fig.2 Q2: The tool shown in Fig.2 is used in a gluing operation to press a thin laminate to a thicker substrate. If the wheels at points A and B both have 2 in...
-
A small country's protectionism can be summarized: The typical tariff rate is 50 percent, the (absolute value of the) price elasticity of demand for imports is 1, imports would be 20 percent of the...
-
a. UHPLC can provide exquisite resolution when run on long columns at high pressure or rapid separations with reasonable resolution if short columns are run fast. The drug acetaminophen run on a...
-
If you were to outsource the whole HR function what reactions would you anticipate from employees and line managers, why do you think they would react like this and what could be done to support them...
-
Patriot Railroad decided to use the high-low method and operating data from the past six months to estimate the fixed and variable components of transportation costs. The activity base used by...
-
The highest rate of return is required by a) Preference shareholders b) Common Shareholders Debt-Holders d) All of the above e) None of the above
-
You have been hired as the new controller for Paulson Paint, Inc. You will have the opportunity to utilize your financial and managerial accounting experience. You will be responsible for preparing...
-
After chlorobenzene has been boiled in water for 2 h, which of the following organic compounds will be present in greatest concentration? (a) C 6 H 5 OH (b) (c) (d) C 6 H 5 Cl (e) Cl HO
-
The transformation of 4-methylbenzenediazonium bromide to toluene is best carried out by using: (a) H + , H 2 O (b) H 3 PO 2 , H 2 O (c) H 2 O, - OH (d) Zn, NaOH
-
Nature Cosmetics Company applies overhead costs on the basis of machine hours. The overhead rate is computed by analyzing data from the previous year to determine the percentage change in costs....
-
In today's stock market, compounding is the key to making money in the future for one's investments. However, with decentralized currency growing rapidly (Crypto), how can one rely on TVM for FV...
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
Write nested for loops to produce the following output: 1 22 333 4444 55555
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
Explain why the ultraviolet spectrum of one of these compounds has its maximum absorption at a longer wavelength than that of the other.
-
Show the structures of the fragment ions that occur at m/z 57, 86, and 99 in the mass spectrum of this compound.
-
Give the ground-state electron configuration for each of the following elements: (a) Oxygen (b) Silicon (c) Sulfur
-
Consumers determine value of the product on the basis of perceived satisfaction the opportunity cost to buy the product discounts availed what other people buy
-
The before-tax income for Grouper Co. for 2020 was $104,000 and $81,200 for 2021. However, the accountant noted that the following errors had been made: 1. 2. 3. Sales for 2020 included amounts of...
-
In Cost / Volume / Profit analysis, variable costs are constant per unit, and the fixed costs are constant in total, over the relevant range. True False

Study smarter with the SolutionInn App



