Which of the following isomeric carbocations is the most stable? CH,+ CH3 () (b) -CH3 CH3 ()
Question:
Which of the following isomeric carbocations is the most stable?
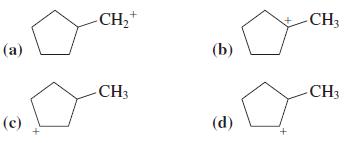
Transcribed Image Text:
CH,+ CH3 (а) (b) -CH3 CH3 (с) (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (14 reviews)
Tertiary Carbocations are the most stable Option a is a primary carbocat...View the full answer

Answered By

Saptarshi Paul
Education--
#Completed my Major in Chemistry in 2018
#Currently pursuing my Master's in Chemistry from IIT Kanpur
Tutoring Experience--
#Taught undergraduate level Chemistry to two students for a period of 6 months.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Which of the following reactions occurs most rapidly? Why? a. b. c. Br - H20 C(CH3)3 C(CH3)a (CH)a C(CH3)3 Br - H20 CH33 C(CH3)3
-
Which of the following compounds can be prepared by radical halogenation with little complication by formation of isomeric by-products? CI CI
-
Which of the following conformers of isobutyl chloride is the most stable Cl CH3 CH, H3C CH3 CH3 CH3 CI
-
On August 31, 2014, Nina Herrera borrowed $5,000 from Second State Bank. Herrera signed a note payable, promising to pay the bank principal plus interest on August 31, 2015. The interest rate on the...
-
Describe the main objective of the Information Distribution process.
-
If your school has a subscription to the FASB Codification, go to http://aaahq.org/ascLogin.cfm to log in and prepare responses to the following. (a) How is common stock defined? (b) How is preferred...
-
Financially distressed firms can gain protection from their creditors while they restructure by filing for protection under US Bankruptcy Codes. In a prepackaged bankruptcy, a firm negotiates a...
-
The City of Clifton provides electric energy for its citizens through an operating department. All transactions of the Electric Department are recorded in a self-sustaining fund supported by revenues...
-
Frost Corporation accepted a $20,000, 8%, 90-day note on July 8. Frost discounts the note on September 6 at East Bank at 9%. What proceeds did Frost receive?
-
Mark is the sole shareholder of Tex Corporation. Mark first formed Tex as a C corporation. However, in an attempt to avoid having Texs income double-taxed, Mark elected S corporation status for Tex...
-
In this transformation, what is the best structure for A? (a) BrCH 2 CH 2 CH(CH 3 ) 2 (b) (c) (d) -, etone A CH;CH,C(CH3)2 H
-
Name the following alcohols according to the IUPAC nomenclature system. Indicate stereochemistry (if any) and label the hydroxy groups as primary, secondary, or tertiary. OH Br OH (a) CH;CH,CHCH; (b)...
-
Would it make sense to connect a fuse or circuit breaker in parallel with other elements in a circuit? Explain.
-
1. Technology and Operations What task does the operations function in a manufacturing organisation and in a service organisation perform? How does operations strategy contribute to make to corporate...
-
Do the Following current market analysis - geographic , psychographic and behavioral of Klean Kanteen THIS IS THE DETAILS AND DRAFTS OF PAPER. (THIS IS THE BASIS) Open the link;...
-
who do you think sets the underlying ethical standards when the law is fuzzy on an issue? as business and societal issues develop in the future, how does your opinion in this area inform your...
-
how do i introduce low risk high reward for a new medical assistant supervisor role in an organization?
-
How do individual differences in cognitive styles, such as analytical versus intuitive thinking, impact problem-solving approaches and decision-making processes within teams ?
-
Write a method called min that takes three integers as parameters and returns the smallest of the three values; for example, a call of min(3, -2, 7) would return -2 , and a call of min(19, 27, 6)...
-
Explain the term "Equivalent Units". Why are they calculated in process costing? [4 Marks] [minimum 350 words]
-
The partial molar volumes of two liquids A and B in a mixture in which the mole fraction of A is 0.3713 are 188.2 cm3 mol-1 and 176.14 cm3 rnol-1 respectively. The molar masses of A and Bare 241.1 g...
-
Estimate the change in the Gibbs energy of 1.0 dm3 of water when the pressure acting on it is increased from 100 kPa to 300 kPa.
-
Calculate the change in the molar Gibbs energy of oxygen when its pressure is increased isothermally from 50.0 kPa to 100.0 kPa at 500 K.
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Under the UCC because a contract for the sale of six freezers does not designate where the goods will be delivered, the place of delivery is the buyer's place of business True or False
-
You just won the TVM Lottery. You will receive $1 million today plus another 10 annual payments that increase by $550,000 per year. Thus, in one year, you receive $1.55 million. In two years you get...

Study smarter with the SolutionInn App


