A mixture of SO2, O2, and N2 from sulfur burning with dry air is fed to a
Question:
A mixture of SO2, O2, and N2 from sulfur burning with dry air is fed to a converter. The mixture contains 8% per volume of SO2. The gas is fed to the converter at 415C and 1 atm. The converter oxidizes SO2 to SO3 using two beds with intercooling. Assume that the equilibrium conversion is reached at each of the beds (which operate adiabatically).
Select between both equilibrium curves below (Fig. P7.1A and B).
Determine the composition of the gases exiting each bed and the cooling temperature after the second. Compute the temperature at which the gases from the first bed must be cooled down to so that the final conversion is 95%.
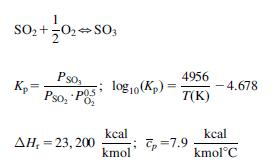

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





