A solution ofNaOH and CaCO3 is to be produced by mixing an alkali solution (84.526% water and
Question:
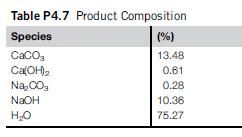
A solution ofNaOH and CaCO3 is to be produced by mixing an alkali solution (84.526% water and the rest NaOH and Na2CO3) with a commercial lime solution ((Ca(OH)21CaO) that contains CaCO3 as an impurity). The product has the composition given in Table P4.7.
Per 100 kg of product, and considering that we have an excess of Ca(OH)2 of 4.45%, determine:
1. The amount and composition of the NaOH1CaCO3 solution.
2. The amount and composition of the commercial lime solution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





