An electrolytic cell is used to produce syngas for ammonia synthesis. Nitrogen comes from air separation while
Question:
An electrolytic cell is used to produce syngas for ammonia synthesis.
Nitrogen comes from air separation while hydrogen is produced from water electrolysis. Hydrogen needs to be compressed up to 300 bar and the oxygen is stored at 125 bar. Assuming perfect water splitting, pure hydrogen and oxygen streams from cathode and anode, respectively, a polytropic (k51.4) efficiency of 85% for the compressors, and the fact that the electrolysis takes place at 80C with a current efficiency of 66%
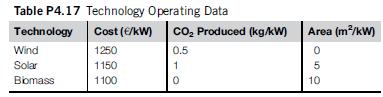
(Table P4.17):
a. Compute the power required to obtain 50 kmol/s of syngas in stoichiometric proportions.
b. Select the appropiate technology.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





