Captured CO2 with nitrogen is to be used as a carbon source for the production of methanol
Question:
Captured CO2 with nitrogen is to be used as a carbon source for the production of methanol (as in the figure). The reaction over Co catalysts is as follows:
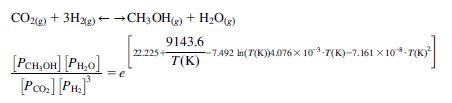
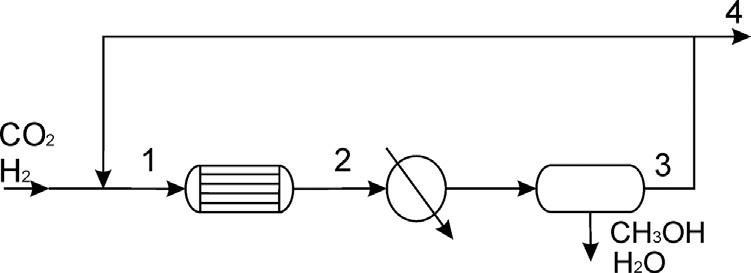
The feed to the system is in stoichiometric proportions containing 0.5% nitrogen. The reactor operates with syngas in stoichiometric proportions at 25 atm. It can handle up to 3 moles of nitrogen per 100 mol of syngas (CO213H2). A conversion of 20% per pass is expected. Assuming that 90% of the methanol and all the water produced are recovered, determine the operating temperature at the reactor and the purge fraction. Fig. P5.24 shows the synthesis loop.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





