FT diesel is produced using hydrogen and CO. The feed gas comes from the partial oxidation of
Question:
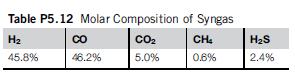
FT diesel is produced using hydrogen and CO. The feed gas comes from the partial oxidation of hydrocarbons. The molar composition is given in dry basis in Table P5.12.
1. This gas is to be processed to obtain a hydrogen-to-CO ratio of 2.
A fraction of the feed is bypassed. The rest is subjected to WGS adding steam. The stream contains 1.6% moles of CO2. Determine the fraction of the gas being bypassed.
2. Next, CO2 and H2S are removed, as well as the water. Compute the moles of CO2 removed from the raw syngas per 100 moles of initial gas.
3. Finally, in a third stage, the syngas produces FT diesel. Determine the operating temperature to maximize the diesel fraction, assuming that the product distribution can be predicted using the AndersonSchulzFlory model. Compute the moles of liquid fuels produced assuming that only the -CH2- synthesis reaction occurs with 70% conversion.
Step by Step Answer:

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín





