Perform the mass and energy balances for the production of HNO3 via catalytic oxidation of NH3. Atmospheric
Question:
Perform the mass and energy balances for the production of HNO3 via catalytic oxidation of NH3.
Atmospheric air (see Table P6.4.1) is mixed with oxygen so that the ratio of oxygen to ammonia becomes 2.15. The ratio of air to added oxygen is 11.5. Assume 1 kmol of atmospheric air for the calculations.
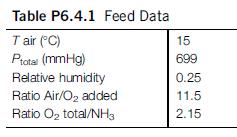
The converter is fed at 36.5C and 699 mmHg. Assume 100%
conversion. Compute the temperature and composition of the gases exiting the converter assuming adiabatic operation. The gases are cooled down to 25C at 699 mmHg. In the process, NO is oxidized to NO2 with a conversion of 15%. Nitric acid is also produced with a composition of 3.5%. The gas is next fed to an oxidation tower.
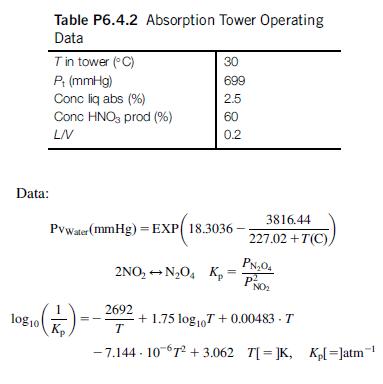
Determine the temperature of the gases exiting the tower assuming adiabatic operation. The gases are cooled down to 30C and fed to the absorptionoxidation tower that operates isothermally. A solution of nitric acid 2.5% is put into contact with the gas. The L/V mass ratio is 0.2. The product gas is expected to reach 60% concentration. Compute the flow of acid solution required (Table P6.4.2).
Step by Step Answer:

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín





