Syngas with an H2-to-CO ratio of 2 is to be produced from C2H2. Steam reforming of the
Question:
Syngas with an H2-to-CO ratio of 2 is to be produced from C2H2. Steam reforming of the hydrocarbon, fed at 900K, is carried out using 50%
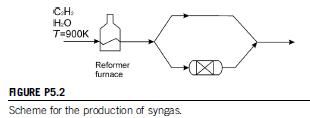
excess of water with respect to the stoichiometric one. In the process, 100% conversion of the hydrocarbon is obtained. A fraction of the gas product is sent to a WGSR, maintaining the temperature from the previous stage, to increase the yield to hydrogen. Assume isothermal operation of the WGSR. See Fig. P5.2 for the scheme of the flowsheet.
Determine the temperature and composition of the gases leaving the reformer, which requires 75,000 kcal/kmol of hydrocarbon and the fraction of the product gas that is fed to the WGSR.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





