The production of a solution of 35% HCl from hydrogen and chlorine is attempted. The gas product
Question:
The production of a solution of 35% HCl from hydrogen and chlorine is attempted. The gas product from the reaction has 95% HCl, and it is absorbed in water (see Fig. P4.14). Compute:
a. The temperature of the converted gases and the energy to be removed from them.
b. The energy removed so that the HCl is fed to the absorption tower at 25C.
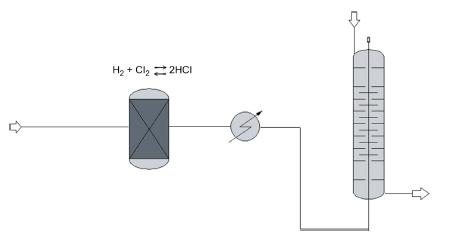
c. The water used in the process.
d. The heat removed for the absorption tower to operate isothermally.
logðkpÞ5 9586 TðKÞ 20:44 logðTðKÞÞ22:16
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín
Question Posted:





