We would like to produce a flowrate of 2 kg/s of hydrogen at 30 bar via water
Question:
We would like to produce a flowrate of 2 kg/s of hydrogen at 30 bar via water electrolysis using different energy sources: solar, wind, or biomass.
The oxygen produced must be compressed up to 100 bar for its storage.
The compressors behave as polytropic, with an efficiency of 85%
and k51.4, and the electrolysis takes place at 80C, consuming 175.000 kJ/kgH2. Assuming ideal water split so that we obtain pure hydrogen from the cathode and pure oxygen from the anode:
a. Determine the energy required for the process.
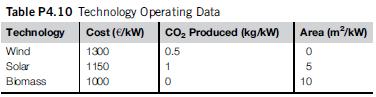
b. Suggest the best technology (see Table P4.10 for the processing parameters).
c. Determine the cost of wind energy so that it becomes competitive, assuming a carbon tax of 40h/t of CO2 and the fact that the ground required costs 25h/m2.
Step by Step Answer:

Industrial Chemical Process Analysis And Design
ISBN: 9780081010938
1st Edition
Authors: Mariano Martín





