In principle, and in the absence of electromagnetic or any other non-gravitational interactions, an electron and a
Question:
In principle, and in the absence of electromagnetic or any other non-gravitational interactions, an electron and a proton should form a ?gravitational atom,? bound by the force of gravity. We know that the energy of the ground state of the ordinary hydrogen atom is given by eq. (14.4)
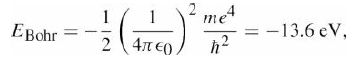
Show that the ground state energy of gravitationally bound ?hydrogen? is given by Egrav = ?1/2G2Nm2pm3e/?2 and evaluate this numerically.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





