Question: Prove analytically that the entropy of a two-statesystem? is maximized when p + = p ? = 1/2. S = -kB (p+ In p+ +p_
Prove analytically that the entropy of a two-statesystem?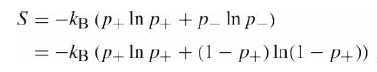
is maximized when p+ = p? = 1/2.
S = -kB (p+ In p+ +p_ In p_) = -kB (P+ In p4 + (1 P+) In(1 P+))
Step by Step Solution
★★★★★
3.29 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Let p x then S k B x ln x 1 x ln1 x so at an ex... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


