Question: Show that roasting lead (2PbS + 3O 2 ? 2 PbO +2 SO 2 ) is an exothermic reaction and compute the free energy of
Show that roasting lead (2PbS + 3O2? 2 PbO +2 SO2) is an exothermic reaction and compute the free energy of this reaction under standard conditions. See Table
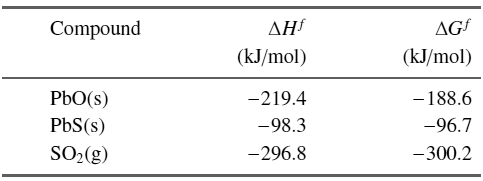
for data.
Compound AG (kJ/mol) (kJ/mol) PbO(s) -219.4 -188.6 PbS(s) -98.3 -96.7 SO2(g) -296.8 -300.2
Step by Step Solution
3.37 Rating (169 Votes )
There are 3 Steps involved in it
Using data from the table the enthal... View full answer

Get step-by-step solutions from verified subject matter experts


