Question: Show that the data in Table 9.6 satisfy the definitions of Gibbs and Helmholtz free energy, (1) ?G r =?H r ? T?S r and
Show that the data in Table 9.6
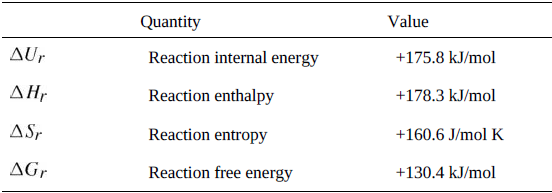
satisfy the definitions of Gibbs and Helmholtz free energy, (1) ?Gr =?Hr ? T?Sr and (2) ?Hr = ?Ur + p?Vr .
Quantity Value AUr Reaction internal energy +175.8 kJ/mol Reaction enthalpy +178.3 kJ/mol ASr Reaction entropy +160.6 J/mol K AGr Reaction free energy +130.4 kJ/mol
Step by Step Solution
3.27 Rating (173 Votes )
There are 3 Steps involved in it
First Second the change in volume is basically ... View full answer

Get step-by-step solutions from verified subject matter experts


