The bond energy of the double bond in the O 2 molecule is 5.06 eV. Verify that
Question:
The bond energy of the double bond in the O2 molecule is 5.06 eV. Verify that light must have wavelength less than???246 nm in order to break this bond. Assuming sunlight has a blackbody spectrum at T = 6000 K,what fraction of the photons in the sunlight incident on the upper atmosphere can dissociate O2? eqs. (22.23)
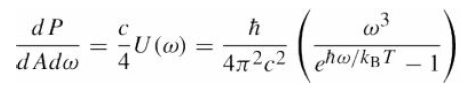
and (22.24)
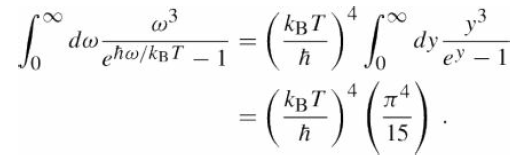
Repeat this calculation for the triple bond in N2, which has a bond energy of 9.61 eV. Compare the relative importance of O2 and N2 as absorbers of ultraviolet light in Earth?s upper atmosphere.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





