Calculate H for the reaction given the following data: NH4(1) + O(g) N(g) + 2HO(1)
Question:
Calculate ΔH for the reaction
![]()
given the following data:
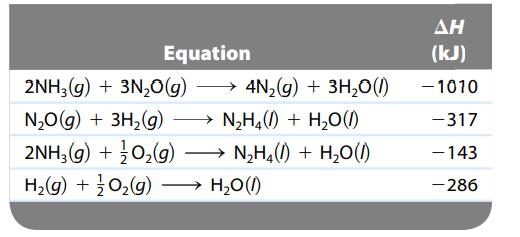
Transcribed Image Text:
N₂H4(1) + O₂(g) N₂(g) + 2H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)

Answered By

Vincent Omondi
I am an extremely self-motivated person who firmly believes in his abilities. With high sensitivity to task and operating parameters, deadlines and keen on instructions, I deliver the best quality work for my clients. I handle tasks ranging from assignments to projects.
4.90+
109+ Reviews
314+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Given the following data: Calculate ÎH for the reaction On the basis of enthalpy change, is this a useful reaction for the synthesis of ammonia? AH - 92 kJ () + AH = -484 k (g) ON OH 88
-
Calculate Go for the following reaction at 25oC. Use data given in Tables 6.2 and 18.1. CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
-
Given subspaces H and K of a vector space V, the sum of H and K, written as H + K, is the set of all vectors in V that can be written as the sum of two vectors, one in H and the other in K; that is,...
-
Suppose treacle is an array of 10 floats. Declare a pointer that points to the first element of treacle and use the pointer to display the first and last elements of the array.
-
Compute the Euclidean distance between the following sets of points: a. (2,5) and (8,4) b. (2, - 1, 32)and (8, 15, - 5)
-
The ammeter in FIGURE P28.52 reads 3.0 A. Find I 1 , I 2 , and . 9.0 V 3.0 ww- 2.0 ww A 3.0 A 1.0 FIGURE P28.52
-
For investments in available-for-sale securities, how are unrealized (holding) gains and losses reported? AppendixLO1
-
A process is in statistical control with x = 202.5 and s = 2.0. Specifications are at LSL = 196 and USL = 206. (a) Estimate the process capability with an appropriate process capability ratio. (b)...
-
At December 3 1 , its annual year - end, the accounts of Sun Systems Inc. show the following. 1 . $ 2 8 8 , 0 0 0 in sales revenue for the year, of which one - sixth was on account. 2 . $ 1 , 4 4 0...
-
Once upon a time many, many years ago, there lived a feudal landlord in a small province of Western Europe. The landlord, Baron Coburg, lived in a castle high on a hill. He was responsible for the...
-
A swimming pool, 10.0 m by 4.0 m, is filled with water to a depth of 3.0 m at a temperature of 20.2 C. How much energy is required to raise the temperature of the water to 24.6 C?
-
Given the following data calculate H for the reaction Fe2O3(s) + 3CO(g) 3Fe,O,(s) + CO(g) Fe3O4(s) + CO(g) 2Fe(s) + 3CO2(g) 2Fe,O4(s) + CO2(g) 3FeO(s) + CO2(g) = = -23 kJ -39 kJ 18 kJ
-
For the purpose of calculating net present value and internal rate of return, do companies use the accrual basis of accounting? Explain.
-
What are electromagnetic moments?
-
How do we design a superconducting synchronous motor?
-
How do we design a superconducting induction motor?
-
How do we design a superconducting cyclo converter?
-
How do we design a superconducting non-inverting op amp circuit?
-
Althea, black, has been a deejay for a local Christian music station for several years. The station got a new general manager and within a month he terminated Althea. The reason he gave was that it...
-
Show that, given a maximum flow in a network with m edges, a minimum cut of N can be computed in O(m) time.
-
Using acetylene and 2-methylpropane as your only sources of carbon atoms, propose a plausible synthesis for 4-methyl-2-pentanone. You will need to utilize many reactions from previous chapters.
-
Draw a Lewis structure of a carbon atom that is missing one valence electron (and therefore bears a positive charge). Which second-row element does this carbon atom resemble in terms of the number of...
-
Below is the structure of caffeine, but its lone pairs are not shown. Identify the location of all lone pairs in this compound: - -N N. .C. Caffeine Z-O- Z-U
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


