Complete and balance each acidbase reaction. a. HPO4(aq) + NaOH(aq) Contains three acidic hydrogens b. HSO4(aq)
Question:
Complete and balance each acid–base reaction.
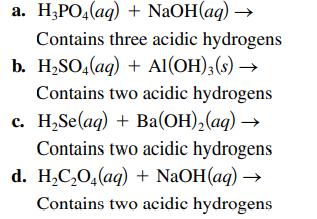
Transcribed Image Text:
a. H₂PO4(aq) + NaOH(aq) → Contains three acidic hydrogens b. H₂SO4(aq) + Al(OH)3(s) → Contains two acidic hydrogens c. H₂Se(aq) + Ba(OH)₂(aq) → Contains two acidic hydrogens d. H₂C₂O4(aq) + NaOH(aq) → Contains two acidic hydrogens
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a H3PO4aq 3NaOHaq Na3PO4aq 3H2Ol b H2SO4aq 2AlOH3s Al2SO43s 6H...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Complete and balance each combustion equation. a. C4H9OH + O2 ( ? b. CH3NO2 + O2 ( ?
-
Complete and balance each combustion equation. a. B2H6 + O2 ( ? (The oxide of boron formed is B2O3.) b. Al2S3 + O2 ( ? (The oxide of sulfur formed is SO2.) c. Al2S3 + O2 ( ? (The oxide of sulfur...
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
1. Prepare a schedule of cost of goods manufactured for Denim Bones for the year ended December 31, 2024. 2. Prepare an income statement for Denim Bones for the year ended December 31, 2024. 3. How...
-
Martha Lou owns 100 shares of Blain Corporation common stock. She purchased the stock on July 25, 1986, for $4,000. On May 2 of the current year, she receives a nontaxable distribution of 100 stock...
-
Majesty Video Production Inc. wants the mean length of its advertisements to be 30 seconds. Assume the distribution of ad length follows the normal distribution with a population standard deviation...
-
Material mix variance is a sub-variance of material price variance.
-
Sears sold a lawn tractor to Cosmo Fiscante for $1,481. Fiscante paid with his personal credit card. Sears kept a valid security interest in the lawnmower but did not perfect. Fiscante had the...
-
1. List the INTERNATIONAL Public Accountancy Firms in Saudi Arabia and the cities where they are located Name of International Audit firms No. Cities Phone No. Email Address 1. 2. 3. (4M) 3. List the...
-
Jenny Smith, 28, just received a promotion at work. Her salary has increased to $40 000 and she is now eligible to participate in her employers pension plan. The employer matches employee...
-
You made 100.0 mL of a lead(II) nitrate solution for lab but forgot to cap it. The next lab session you noticed that there was only 80.0 mL left (the rest had evaporated). In addition, you forgot the...
-
In the spectroscopic analysis of many substances, a series of standard solutions of known concentration are measured to generate a calibration curve. How would you prepare standard solutions...
-
Who is already informed about the project?
-
8 Project two 15 UTSA Project two M Question 1 - Project two ChatGPT C chegg.com/homework-he X Course Hero how to take a sxreen shot X +...
-
Charitable purposes: Section 3(1) Charities Act 2011 1. Prevention or relief of poverty 2. Education 3. Religion, now includes: 4. - - A religion which involves belief in more than one god A religion...
-
Jack Price, The finance director of Humpty Doo Investment Ltd ( HDIL ) , is unsure whether he should consolidate some of the investments that the company owns. He has asked your advice as business...
-
Use QM to solve this problem. Suppose that Peter Cartman is deciding whether to invest in a bond mutual fund or a stock fund. Both bond and stock funds are sensitive to changing market conditions....
-
George Francis works at Gentry Medical Center which is in sunny Florida. The Medical Center experiences a higher volume of business closer to fall when many of the patients return for the winter from...
-
Draw up a similar table to the one above, only this time assume that the basic benefit is 6000. Assume that the marginal tax rate is 20 per cent up to 10 000 and 40 per cent from 10 000 to 20 000. To...
-
A fuel pump sends gasoline from a car's fuel tank to the engine at a rate of 5.88 10-2 kg/s. The density of the gasoline is 735 kg/m3, and the radius of the fuel line is 3.18 10-3 m. What is the...
-
Which of the following molecules may show a pure rotational Raman spectrum? (a) CH2Cl2 (b) CH3CH3, (c) SP6, (d) N2O?
-
An object of mass 2.0 g suspended from the end of a spring has a vibrational frequency of 3.0 Hz. Calculate the force constant of the spring.
-
Calculate the percentage difference in the fundamental vibration wave number of IH35Cl and 2H37CIon the assumption that their force constants are the same.
-
The following amounts were reported on the December 31, 2022, balance sheet: Cash $ 8,000 Land 20,000 Accounts payable 15,000 Bonds payable 120,000 Merchandise inventory 30,000 Retained earnings...
-
Sandhill Co. issued $ 600,000, 10-year, 8% bonds at 105. 1.Prepare the journal entry to record the sale of these bonds on January 1, 2017. (Credit account titles are automatically indented when the...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.

Study smarter with the SolutionInn App


