Consider the following mixture of SO 2 (g) and O 2 (g). If SO 2 (g) and
Question:
Consider the following mixture of SO2(g) and O2(g).
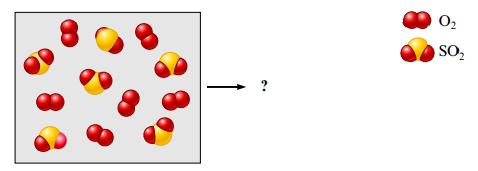
If SO2(g) and O2(g) react to form SO3(g), draw a representation of the product mixture assuming the reaction goes to completion. What is the limiting reactant in the reaction? If 96.0 g of SO2 react with 32.0 g O2, what mass of product will form?
Transcribed Image Text:
? 0₂ SO₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a The limiting reactan...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What is a limiting reactant in a reaction mixture? Explain how it determines the amount of product.
-
What is a limiting reactant problem? Explain the method you are going to use to solve limiting reactant problems.
-
Describe how you could separate the following mixture of metal ions: Cd2+, Pb2+, and Sr2+.
-
The number x of bicycle helmets people are willing to buy per week from a retail chain at a price of $p is given by x = 1,000 - 60p + 25 20 ¤ p ¤ 100 (see the figure). (A) Find dx/dp....
-
Because expenses incurred both in a business and for the production of investment income are deductible, why is it important to determine in which category a particular activity falls?
-
The screw of the clamp exerts a compressive force of 500 lb on the wood blocks. Sketch the stress distribution along section aa of the clamp. The cross section is rectangular, 0.75 in. by 0.50 in. 4...
-
Consider the model: E1y2 = b0 + b1x1 + b2x2 + b3x2 2 + b4x3 + b5x1x2 2 where x2 is a quantitative model and where x1 = e 1 received treatment 0 did not received treatment The resulting least squares...
-
The demand for a product of Carolina Industries varies greatly from month to month. Based on the past two years of data, the following probability distribution shows the companys monthly demand: Unit...
-
finanicail Accounting On June 30, 2014, Diode Inc. .26 purchased for cash at $50 per share all 150,000 shares of outstanding common stock of Moore Company. Moore's balance sheet at June 30, 2014,...
-
Sara-Jayne Parsons is an architect who operates her own business. The accounts and transactions for the business follow. INSTRUCTIONS 1. Analyze the transactions for January 20X1. Record each in the...
-
You know that chemical A reacts with chemical B. You react 10.0 g A with 10.0 g B. What information do you need to determine the amount of product that will be produced? Explain.
-
Consider an iron bar on a balance as shown. As the iron bar rusts, which of the following is true? Explain your answer. a. The balance will read less than 75.0 g. b. The balance will read 75.0 g. c....
-
Derive the expression for the stress induced in a shaft with an unbalanced concentrated mass located midway between two bearings.
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
1. Under what circumstances would the interdependence of firms give rise to a vertical long-run AS curve? 2. If a shift in the aggregate demand curve from AD 1 to AD 2 in Figure 3 causes a movement...
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
Prove that the perfect gas temperature scale and the thermodynamic temperature scale based on the Second Law of thermodynamics differ from each other by at most a constant numerical factor.
-
Evaluate (ClS/ClV)]' for (a) A van der Waals gas, (b) A Dieterici gas (Table 1.7). For an isothermal expansion, for which kind of gas (and a perfect gas) will /).5be greatest? Explain your conclusion.
-
Two of the four Maxwell relations were derived in the text, but two were not. Complete their derivation by showing that (S/V)T = (p/T)V (T/P)s = (V/S)p
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....

Study smarter with the SolutionInn App


