Consider the following two acids: In two separate experiments the pH was measured during the titration of
Question:
Consider the following two acids:
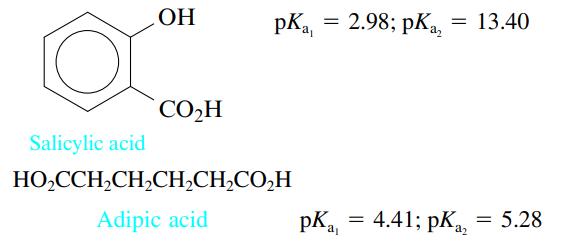
In two separate experiments the pH was measured during the titration of 5.00 mmol of each acid with 0.200 M NaOH. Each experiment showed only one stoichiometric point when the data were plotted. In one experiment the stoichiometric point was at 25.00 mL added NaOH, and in the other experiment the stoichiometric point was at 50.00 mL NaOH. Explain these results.
Transcribed Image Text:
OH CO₂H = pKa, = 2.98; pka₂ : 13.40 Salicylic acid HO₂CCH₂CH₂CH₂CH₂CO₂H Adipic acid pK₁ = 4.41; pKa a₂ 5.28
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (14 reviews)
Solution The results explanation entails an understanding of the concept of equivalence point ...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider the following two independent situations: 1. A manufacturer reported an inventory turnover ratio of 8.6 during 2013. During 2014, management introduced a new inventory control system that...
-
Consider the following two samples: Sample 1: 10, 9, 8, 7, 8, 6, 10, 6 Sample 2: 10, 6, 10, 6, 8, 10, 8, 6 (a) Calculate the sample range for both samples. Would you conclude that both samples...
-
Consider the following two mutually exclusive projects: Whichever project you choose, if any, you require a 15 percent return on your investment. (a) If you apply the payback criterion, which...
-
3. (15 pts.) The random variables X and Y have joint density function fxx(x, y) x-?y?, x > 1, y > 1. Compute the cdf and pdf of the random variable U = XY.
-
Art Wyatt Pool Company wishes to finance a $15 million expansion program and is trying to decide between debt and external equity. Management believes that the market does not appreciate the...
-
Why is a table whose primary key consists of a single attribute automatically in 2NF when it is in 1NF?
-
What, if any, difference do you think it will make in the way Mindy and Stacey recruit new staff members for their property if they are no longer affiliated with the chain organization?
-
A partnership of attorneys in the St. Louis, Missouri, area has the following balance sheet accounts as of January 1, 2018: Assets . . . . . . . . . . . . $320,000 Liabilities . . . . . . . . . ....
-
FULL SCREEN PRINTER VERSION BACK NEXT Question On Anni 7, 2021, Mos Motor Corp. had a balance of $420,000 in its Common Shares account and the total number of shares sued was 39,000. On April, 2021,...
-
Estelle owns a large concreting business as a sole trader. The business operates ten concreting trucks, each worth approximately $200,000, five of which Estelle holds freehold. Estelle would like to...
-
The common ion effect for weak acids is to significantly decrease the dissociation of the acid in water. Explain the common ion effect.
-
Sketch the titration curves for a diprotic acid titrated by a strong base and a triprotic acid titrated by a strong base. List the major species present at various points in each curve. In each...
-
Antigens for a parasitic roundworm in birds. Ascaridia galli is a parasitic roundworm that attacks the intestines of birds, especially chickens and turkeys. Scientists are working on a synthetic...
-
Dr. Stanley and his staff are attempting to utilize effective ways to both increase the revenue for the practice and allow patients to schedule visits without a lengthy delay. Which of the following...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Centurion Inc. manufactures lighting equipment. It consists of several operating divisions within its business. Division A has decided to go outside the company to purchase materials since Division B...
-
Meta has also reduced its operations, and instead focused on retaining wealth for research and development, as well as increasing shareholder returns...What does this mean for the company's future?
-
Please answer the following question short and simple: Tom Anderson is the controller for Morningside Medical Clinic. At the end of each month, the financial management system used by Morningside...
-
Find the exact value of expression. sin sin + cos 0 2
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
Assume you are carrying out the dehydration of 1-methylcyclohexanol to yield 1-methykyclohexene. How could you use infrared spectroscopy to determine when the reaction is complete?
-
Assume that you are carrying out the base-induced de-hydro bromination of 3-bromo-3-methylpentane (Section 11.7) to yield an alkene. How could you use IR spectroscopy to tell which of two possible...
-
Which is stronger, the C = O bond in an ester (1735 cm1) or the C = O bond in a saturated ketone (1715 cm1)? Explain.
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


