For a temperature of 300C and a lifetime of three years, what is the maximum stress that
Question:
For a temperature of 300°C and a lifetime of three years, what is the maximum stress that the nickel-based component of Figure 7-31 can withstand?
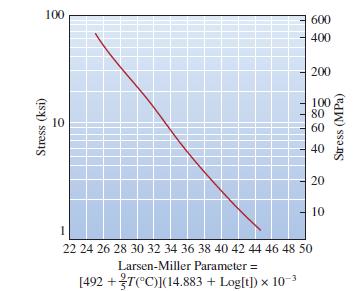
Transcribed Image Text:
100 Stress (ksi) 10 600 400 200 100 80 60 40 20 10 1 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 Larsen-Miller Parameter = [492 +T(°C)](14.883 + Log[t]) x 10-³ Stress (MPa)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
The maximum stress 299 MPa The m...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
For a temperature of 300 K, use the equation in Problem 49 to find the bias voltage Vb for which the exponential term has the value (a) 10 and (b) 0.1.
-
What is the maximum taxable wage?
-
What is the maximum electric energy density possible in dry air without dielectric breakdown occurring?
-
which you describe and reflect on one of your favorite pop culture artifacts. Your goals will be simple: you will describe in detail a popular film, tv show, ad campaign, performer, song,...
-
Explain how a personal guarantee reduces the limited liability of the principals of a closely held corporation.
-
On February 11, 20Y9, Quick Fix Company purchased $2,250 of supplies on account. In Quick Fixs chart of accounts, the supplies account is No. 15, and the accounts payable account is No. 21. a....
-
Mymanagementlab Only comprehensive writing assignment for this chapter. (p. 208)
-
At the market close on October 27 of a recent year, McDonalds Corporation had a closing stock price of $93.49. In addition, McDonalds Corporation had a dividend per share of $2.44 during the previous...
-
***Please solve using simple regression and NOT high-low method. Thanks!*** Allison, the owner of Accountants Inc., is trying to forecast overhead costs for the next month. Allison estimates that...
-
Newlyweds Jamie Lee and Ross have had several milestones in the past year. They are newly married, recently purchased their first home, and now have twins on the way! Jamie Lee and Ross have to...
-
A 1-in-diameter bar of an iron-chromiumnickel alloy is subjected to a load of 2500 lb. How many days will the bar survive without rupturing at 980C?
-
A nickel-based alloy component (INCONEL alloy 601) is designed to withstand a service stress of 4000 psi at a temperature of 925C. Determine the temperature at which the component should be operated...
-
In Problems 4374, find the real solutions of each equation. 04 5x2 = X
-
how could playing in a sandbox help to the development of children? how could a garden help to the development of children? how could playground obstacle courses like a pebble bridge and monkey bars...
-
A store order bottles of shampoo throughout the year. Over time, the store has learned that the annual demand D for shampoo is constant, i.e., there is no variability. Currently, the store decides to...
-
Solve the Practice #2 == where L2 =02A = a, L404B = c, L = 0204 = d, y = /2 1) Find the velocity 3 when 82 = /2 and 6 = 0.4 rad/s 2) Find the acceleration 63 when = /2 and 62 = 0.4 rad/s 03. 03 Y B...
-
.0.5 0.5 For the above plot of the ellipsoid (22) 2- + +() + (-) = 1, find the parameters a, b and c. Note that a, b and c are positive integers between 1 and 6 inclusive. Use the mouse to rotate the...
-
The annual energy consumption of the University of Maryland is 100 million kWh. How much Uranium-235 is needed to produce this amount of energy in a nuclear power plant assuming 100% efficiency? (The...
-
True or False. If (r, ) are polar coordinates, the equation defines a hyperbola. 2 + 3 sin 0 2.
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
Hydrogen reacts explosively with oxygen. However, a mixture of H 2 and O 2 can exist indefinitely at room temperature. Explain why H 2 and O 2 do not react under these conditions.
-
The rate of the reaction depends only on the concentration of nitrogen dioxide below 225 C. At a temperature below 225 C, the following data were collected: Determine the rate law, the integrated...
-
The type of rate law for a reaction, either the differential rate law or the integrated rate law, is usually determined by which data is easiest to collect. Explain.
-
Be prepared to explain the texts comprehensive To illustrate the issues related to interest capitalization, assume that on November 1, 2016, Shalla Company contracted Pfeifer Construction Co. to...
-
On April 1, 2020. Indigo Company received a condemnation award of $473,000 cash as compensation for the forced sale of the company's land and building, which stood in the path of a new state highway....
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...

Study smarter with the SolutionInn App


